Shell shocked: Emerging impacts of our acidifying seas
Our changing climate is altering the chemistry of the ocean, and some animals are paying the price

Corals provide the base of a unique community of ocean creatures. But ocean acidification may make it difficult for corals to build their beautiful structures.
iBorisoff/iStockphoto
This is the seventh in a 10-part series about the ongoing global impacts of climate change. These stories will look at the current effects of a changing planet, what the emerging science suggests is behind those changes and what we all can do to adapt to them.
Pry open your first oyster shell, and the vision that greets you isn’t that appetizing. The grey, shiny glob inside looks cold and slimy. But people the world over are obsessed with eating this shelled delicacy.
“The most popular way to eat them in Australia is raw,” says Elliot Scanes. He’s a marine biologist at the University of Sydney in Australia. While oysters may be slimy and “have the texture of a booger,” he notes, the flavor is a winner with a lot of people. Oysters, he says, “taste like salty sushi.” And there’s big business in these ocean boogers.
In the U.S. Pacific Northwest, oysters bring in $111 million per year. In Australia, where Scanes is a marine biologist at the University of Sydney, it’s $26 million.
But those millions — and the oysters that bring them in — are in danger. In the Pacific Northwest, baby oysters have died off by the billions. Their tiny shells dissolved before they were fully formed. In Australia, Scanes finds there are more female and fewer male Sydney rock oysters — which could affect how many oysters fill the plates of future Australian diners.
The cause is ocean acidification. Through their industrial activities and the burning of fossil fuels, people have been pumping more and more greenhouse gases, such as carbon dioxide, or CO2, into the air. The ocean will absorb about one-fourth of that gas. There, it will react with water in ways that make the ocean slightly more acidic.
People might never notice the change when going for a swim, but to organisms that call the sea home, acidification is a source of stress. It puts many of the seafoods we love to eat — such as oysters — at risk. And the only way to stop it is to stop the release of CO2 into the air.
The ocean: So basic
Today’s ocean is slightly basic, or alkaline. Scientists use the 14-point pH scale to classify substances from very acid to very alkaline. Pure water is a perfectly neutral 7. Battery acid falls around 1. Soapy water is about 12.
Water in the ocean contains salt (sodium chloride) and other chemicals such as calcium and boron. Water molecules themselves are always morphing. The H2O breaks into negatively charged hydroxide ions (oxygen and hydrogen) and positively charged hydrogen ions. Those hydrogen ions are the very definition of an acid. (An acid is any substance that releases hydrogen ions.) A lot of extra hydrogen atoms makes a substance acidic.
Some of those ions react with the other chemicals in water. If hydrogen ions outnumber hydroxide ions, water is somewhat acidic. If hydroxide ions outnumber hydrogen ions, it’s more basic. Ocean water is slightly basic, with a pH around 8.2.
Or at least, that’s what it used to be.
Around one-fourth of humanity’s emissions of CO2 are being absorbed by the world’s oceans. There, this gas reacts with water molecules and carbonate ions (one carbon and three oxygen atoms). The result is a chemical called bicarbonate — and a bunch of leftover hydrogen ions. Those hydrogen ions add to the hydrogen ions already present, making the seawater more acidic.
Since the mid-18th century, when people started burning fossil fuels in huge amounts, the pH of the global ocean has dropped by a tenth of a point, notes Nyssa Silbiger. She studies ocean acidification at California State University in Northridge. That tenth of a point may seem small. But pH is measured on a logarithmic scale, where each additional 1 point is equal to a 10-fold increase. A substance with a pH of 8 has 10 times the amount of hydroxide ions as one with a pH of 7. A change from 8.2 to 8.1, then, isn’t so small.
But this change is not the same all over the ocean. The 0.1 point change, Silbiger notes, is an average over the whole, huge ocean. It can be somewhat higher or lower, depending on the time and place.
Chemical rollercoasters
Studying pH in the vast, open ocean isn’t easy. Some scientists instead set up experiments in the lab. Silbiger, though, turned to a natural experimental space: California’s coastal tide pools.
These are divots in the beach that get cut off from the sea at low tide. But they collect water as ocean waves wash over them during high tide. The pH of water in these shallow pools seesaws up and down. In a single day, a tide pool’s pH can swing from a low of 7.09 to a high of 8.90. At the same time, the temperature will vary as the pool is cut off from the ocean and warmed by the sun. Even the pool’s oxygen levels rise and fall. It can make for a tough lifestyle. Still, many organisms — such as starfish, sea urchins and algae — thrive here.
Tide pools are an excellent opportunity for science. “When [a tide pool is] separated from the ocean,” Silbiger says, “I’m able to count every single organism” and study the water’s chemistry. In this way, she can figure out “how the organisms relate to changes in the chemistry.”

Piling groceries on top of lab equipment, Silbiger and another scientist spent two months road-tripping up the West Coast of the United States in a camper van. On their trip, they tested tide pool after tide pool.
The most important factor for a tide pool’s pH was what was living in it, Silbiger found. Algae, seagrass and other photosynthetic organisms power their activity with sunlight by day. Along the way, they use up CO2. The result is an increase in pH — meaning the water becomes less acidic. At night, when there’s no sun, these organisms put CO2 back into the water. That brings the pH back down — making it more acidic.
Animals in tide pools produce CO2 all the time, day and night. That CO2 acidifies the tide pool. The more photosynthetic organisms living in a tide pool, the more it pH goes up by day. The more animals in the tide pool, the more its pH goes down at night.
Silbiger and her colleague Cascade Sorte, of the University of California, Irvine, published their findings January 15, 2018 in Scientific Reports.
This daily pH rollercoaster washes over the organisms living in the tide pools and nearby shallow areas of the sea. If you’re a mussel, clam or other organism with a hard shell, these pH changes can’t be ignored.
Coming out of your shell
The ocean isn’t a place you’d want to live without protection. Waves smash. Water presses in on all sides. Hungry predators roam.
Mussels, oysters, coral and many other organisms in the ocean protect themselves with a tough shell. It’s formed from calcium carbonate. This chemical has one atom of calcium hooked to a carbonate ion.
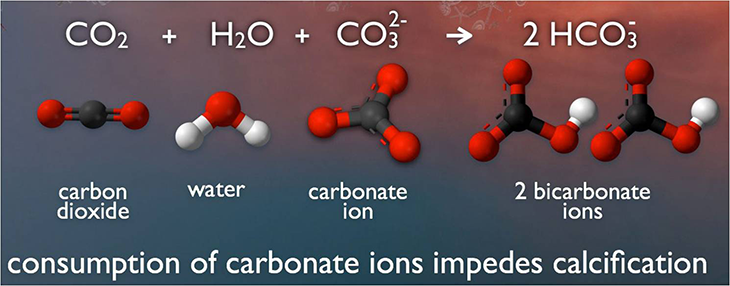
Under normal ocean conditions, a critter can make enough calcium carbonate from calcium and carbonate in the water to build its shell. But as more CO2 from the atmosphere dissolves into the ocean, more bicarbonate forms. That uses up carbonate, leaving shell-building organisms with less shell-building calcium carbonate.
That shortage can affect everything from how a coral grows to how an oyster dates.
Corals and oysters aren’t great at talking about their feelings. But Emily Rivest can tell they are stressed. She’s a marine biologist at the Virginia Institute of Marine Science at the College of William and Mary in Williamsburg. There, she and her students deploy sensors and fill aquariums with oysters from the nearby Chesapeake Bay.
Mussels, oysters and other tide-pool denizens suffer in a more acidic ocean, Rivest has found. By day, as plants and other photosynthetic organisms are raising the tide pools’ pH, shell-making organisms build away. At night, as the pH drops, shell-building does too. And the lower the pH gets at night, the less building that takes place, Rivest’s team has shown. Oysters can handle the wild tidepool pH swings. But only to a point. If the pools don’t get basic enough during the day, they can’t build up enough shell.
The term “ocean acidification” brings up visions of acid water eating away at a poor, defenseless oyster. But that’s really not the case, Rivest says. “It’s not that they’re getting small because the oysters are dissolving from the outside in,” she explains. “It’s because they’re growing slower from the inside out.”
Growth slows because the oyster has to spend more energy to pull calcium and carbonate out of the water to build its shell. Ocean acidification is making the animal work harder.
“It would be awesome to see an oyster running around looking for calcium carbonate, but they’re stuck in one spot,” Rivest says. “They have to spend more energy to lay down the shell.” Making up that energy means getting more to eat — or just growing less.

The future is female
Ocean acidification can affect oysters in other ways as well. In fact, it affects how oysters mate — and make more oysters, finds Scanes of the University of Sydney.
After oysters mate, male oysters are born. But female oysters aren’t born. They’re made. After oyster larvae float around in the water and settle on a surface, they spend their first year growing. For that first year, oysters grow male gonads, or reproductive organs. Then they’ll spawn for the first time as males, releasing clouds of sperm into the water.
Oysters re-grow their gonads every year. By the second year, some of these oysters now grow a female’s egg-making gonads. After a while, if the oysters aren’t too crowded, Scanes says, about 50 percent of the oysters will become females that yearly release eggs.
But if the ocean continues to acidify, the future for oysters looks increasingly female.
In an experiment, Scanes and his colleagues added more CO2 to the water that their oysters lived in. It was to see what life in a more acidic sea might look like to these shellfish. They added enough CO2 to bring the pH down to 7.9. That pH is in the range the Intergovernmental Panel on Climate Change predicts could occur in 100 years if people continue to emit greenhouse gases the way we do now.
If oysters were exposed to a newly acidic ocean for two months before their next spawning, they were more likely to end up female, Scanes’ team found. “After just these 8 weeks, [there] were 16 percent more females,” he says. If that trend continues, with a greater share of the oysters now female, fewer baby oysters may result.
Scanes and his colleagues published their results February 14, 2018 in the Proceedings of the Royal Society: B.
Oysters are important because we eat them. But their value extends beyond that. Oysters, mussels and other shelled species are filter feeders, cleaning the water as they eat. They’re also foundation species — ones that build structures that other organisms can hang out in.
The best-known foundation species might be corals. They, too, create their elaborate structures from calcium carbonate. “Coral builds a complex three-dimensional reef structure that provides a home to dozens of other species,” Rivest notes. “Without that structure, you don’t have those benefits.”
California Academy of Sciences/YouTube
It takes a coral village
Ocean acidification can affect the growth of a single coral. But these organisms are not loners. Corals pack together, producing reefs that in turn provide housing and food for other creatures. These reefs function as huge ocean cities.
As seas acidify, corals might not be the only organisms affected in those cities. There’s a whole community of fish, microbes, invertebrates and other things that live on and near these corals. They, too, might be affected.
This isn’t something that can be easily studied in a lab or tide pool. So Rebecca Albright flew halfway around the world. She is a coral-reef biologist at the California Academy of Sciences in San Francisco. Albright was part of a team that investigated this at the world’s largest coral community — the Great Barrier Reef.
Doing research here, off the coast of Australia, is not very glamorous. At the research station on One Tree Island, all food has to be brought in by plane and then by boat. Lettuce arrives so rotted it can drip out of the bag. Scientists staying there “shower” with buckets of sea water.
But it has all been worth it to turn the ocean pink.
Albright and her group began with a small swimming pool in the ocean. The team collected 15,000 liters (almost 4,000 gallons) of seawater, enough to fill the pool. They added pink dye and bubbles of carbon dioxide (CO2). “We just bubbled it up like a massive SodaStream,” Albright says. The gas and coloring dissolved into the seawater, making it more acidic.
Then Albright and her colleagues released the pink sea water onto the reef. This lowered the pH in a small area. Every third day for two months, the scientists decreased the pH this way for an hour. The change was small, a drop from 8.1 to 8.0. But that lower pH slowed the reef’s building rate by up to one-third. This was a much bigger drop than most studies find when they look at reefs in the lab, Albright points out.

“That’s really concerning,” she adds, “because coral reefs are being hammered by bleaching.” Bleaching is what happens when corals get too warm. The heat can make them evict the helpful algae that normally live inside them. That leaves the corals bone-white. The corals aren’t dead, at this point. But they are stressed. To recover, the corals must grow quickly, Albright says. But her data show that “acidification is slowing that process.”
Albright and her colleagues published their results March 14, 2018 in the journal Nature.
To make things more complex, not every coral reef responds the same way.
Andreas Andersson is an oceanographer at the University of California at San Diego. He works on reefs in clear, blue seas off Bermuda, on the other side of the planet from the Great Barrier Reef. Bermuda’s reefs sit on the edge of the tropics. That means they experience more ups and downs in temperature than tropical reefs. Different types of corals hang out here, too. “If you look the reefs in Bermuda, [they’re] different from reefs you find closer to the equator,” says Andersson. “[They have] more massive corals, not branching corals.”
Andersson has found that Bermuda’s reefs don’t respond to ocean acidification as those do in Australia. “They seem to be pretty strong,” he says. “We’ve done experiments exposing them to very high acidity levels. And they don’t seem to care.” In fact, as winters get warmer, the Bermuda corals grow faster. Because of this, Andersson hypothesizes that reefs like those in Bermuda could be what coral reefs of the future may come to look like.
Or not. “It could also be that the [reefs] in Bermuda [have] been lucky,” Andersson admits. And if the waters get too warm, even the tough Bermuda corals will bleach.
Back to the future
Sometimes, to find out how the future might look, it helps to look back at the past. That’s why Carrie Lear and Sindia Sosdian are looking at evidence from ancient oceans. It offers to tell them about how ocean acidification might change seas of the future.

Lear studies paleoclimate (“paleo” refers to the past). Sosdian studies climate change. Both work at Cardiff University in Wales. To trace the pH of oceans past, the two have been looking at sediment dug up from the seafloor. Littering the sediment are traces of single-celled sea critters. They’re known as foraminifera (For-am-in-NIF-er-uh), or forams.
Forams have two traits that make them great for studying acidification. One: They’ve been around a long time. Some species of them have been floating through the ocean for millions of years. More importantly, they have tiny, calcium-carbonate shells. They build up those shells just as oysters and corals do. But when they slurp up calcium carbonate, they’re not very picky. They’ll sometimes take in other molecules too. Such as borate ions. These are boron atoms bonded to three oxygen atoms.
Forams take up borates as they build their shells. So Lear and Sosdian looked at tiny shells from around the world and going back millions of years. At different times, different boron isotopes — versions of an element with different numbers of neutron particles in their nuclei — prevailed. How much boron forams slurp up differs when the pH of the ocean goes up or down. So the amount and type of boron in foram shells told them how acidic the ocean had been at various times in the past.

Lear and Sisdian were most curious about when in the past the pH of the ocean was last as low as 7.8. That’s what the ocean’s pH might be 100 years from now, If people don’t reduce how much CO2 they pump into the atmosphere. Lear and Sosdian’s forams showed the ocean’s pH was last that low 14 million years ago — a time called the Miocene (MY-oh-seen) climate optimum.
“In the Miocene climate optimum, CO2 was higher than today,” Sosdian now reports. “But it was more of a natural phenomenon.” There was a rise in volcanic activity at this time, she explains. That threw a lot of CO2 into the air and sea. Sosdian and Lear published their findings September 15, 2018 in Earth and Planetary Science Letters.
“That’s kind of a ‘wow’ moment,” Sosdian notes, “to say we are coming to a point we haven’t seen” for 14 million years. “We’re getting an appreciation for how this will impact marine life.”
When the Miocene ocean acidified, some corals and mollusks died out. Others soldiered on. Some plankton — the small organisms that drift through the sea — used calcium carbonate for their shells. They didn’t just survive, they truly thrived.
However, the last time the seas were this acidic, the process had occurred slowly. So species had millions of years to adapt. What took millions of years then may now develop in only a few decades. And most species likely won’t be able to adapt that quickly.
So, how can we stop ocean acidification to save oysters and coral reefs? “It’s the hardest question!” Albright says. Some people have suggested dumping chemicals into the ocean to make it less acidic. Others are developing corals that can resist more acidific water.
“There are lots of things being looked at to try and help reefs over the next 50 to 100 years,” she says. “But I’ve not met a single person in the scientific community who feels that’s going to solve this problem.” If we continue to dump CO2 into the air and ocean at our current pace, she notes, no amount of resistant coral or pH-raising chemicals will stand up to our efforts.
“The only solution,” Albright says, “is stopping [climate change] in the first place.”
Correction: This article was revised on February 28, 2018 to correct the identity of the colleague traveling with Nyssa Silbiger.







