Snail slime + gold could boost the power of sunscreens and more
The pair could make skin-care products that are better for our skin and the environment

Snail-slime skincare products and cosmetics have been around for more than 1,000 years. But adding gold nanoparticles could give these personal-care products a new edge.
IrenaV/iStock/Getty Images Plus
Two unlikely materials — snail slime and gold — may be the secret to better sunscreens.
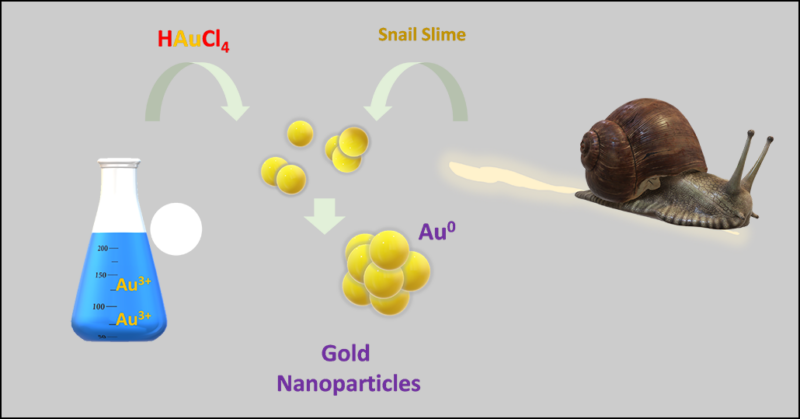
Suncreens often contain tiny particles that reflect sunlight. Usually those are bits of the metals zinc or titanium. But nanoparticles of gold could work better due to their high reflectivity and low toxicity. To make such nanobits, researchers in Italy used snail slime to clump gold atoms together. The mucus made the process eco-friendly and also could help personal-care products last longer.
The researchers hope their slimy gold specks will be used one day in sunscreens and other cosmetics. And these innovative products may not only be great for our skin but also safer for our planet.
The study describing the new tech appeared in the November Journal of Photochemistry and Photobiology B: Biology.
Spas and snails and slimy trails
Snail slime might sound like the last thing you’d want to smear on your face. But snail-based skincare is far from new. Even the ancient Romans used it. Today, some spas offer “snail facials.” Live snails ooze across the face. The goopy trails they leave can hydrate skin. They also can apparently lighten age spots.
For their new study, researchers in Bari, Italy, used slime that had been harvested from the brown garden snail (Helix aspersa Müller). A special ozone-filled chamber had encouraged the snails to ooze slime. “But in a mild way,” adds Pinalysa Cosma — “not in a way that hurts the snails.” Cosma is a chemist on the team. She works at the University of Bari Aldo Moro.
Her team mixed that slime into a flask with gold atoms dissolved in water. Snail slime changed those gold atoms from one form, called Au3+, into a type called Au0.
“The main component of snail slime — a protein — is a reduction agent,” explains Vito Rizzi. He’s another chemist on the team at the University of Bari Aldo Moro. A reduction agent, he explains, gives up electrons to chemical ions.
When the snail-slime protein gives electrons to Au3+, the gold is no longer an ion. It turns it into Au0, Cosma says, which “is the metal form of gold.” Unlike Au3+, this form doesn’t dissolve in water. So individual atoms of this gold now clump into nanoparticles.
Companies often make gold nanoparticles with chemicals that are “not eco-friendly,” Rizzi notes. Those chemicals can release pollutants into the environment. Snail slime doesn’t. (Other natural substances have also been used as a “greener” way to make nanoparticles, such as extracts from plants or fungi.)
Sunscreen of the future?
Gold is really shiny. Imagine a bright gold coin gleaming in the sun. The gold bits reflect sunlight like nanomirrors. (So do other metals. That’s why the back of your sunscreen bottle might list “zinc oxide” or “titanium dioxide” among the ingredients.) But gold reflects more light than reflective ingredients currently used in sunscreens.
Snail slime itself also appears to boost gold’s sun-screening abilities. This is probably because the mucus absorbs some light at the same time the gold is reflecting light. Many sunscreens contain ingredients that do both.
Cosma’s team dissolved its nanogold in water and then measured how much light the mix absorbed. Compared with plain gold nanoparticles, the team’s snail-slimed particles absorbed more. That’s important. It means adding slime-coated gold could boost a sunscreen’s protection even more than plain gold bits. This should give sunscreens a higher sun-protection factor, or SPF.
Snail slime offers another benefit, too. It boosts the gold nanoparticles’ ability to mop up harmful chemicals called oxygen radicals, also known as oxidants. Many normal chemical reactions in the body produce these radicals. But too many can cause problems. For example, they can kill cells. Oxygen radicals also can react with and break down sunscreen ingredients. That’s why companies add antioxidant chemicals to to fight the radicals. The Italian study reports its nanoparticles also act as antioxidants.
“This is a very good study,” says Bipinchandra Salunke. He’s a biotechnologist in Dhule, India (where he’s currently helping to develop a start-up company). Salunke was not involved in the new research, but he has made gold nanoparticles in the past. Back in 2014, he was part of a team that used a plant extract to do that. His group described its technique in the International Journal of Cosmetic Science.
The Bari group is “adding one new biological agent to the library,” Salunke says. “And they are showing biomedical applications, also.” He refers to a previous study by the Bari team. It showed that slimy gold nanoparticles could help wounds heal faster. The researchers reported those results in the December 2020 issue of Soft Matter.
Challenges to overcome
The slime-gold duo may not be ready for widespread use just yet. The reason: A particle’s structure affects which chemicals it can react with. So nanoparticles of different shapes and sizes can have different traits. Some nanogold bits may work better for particular uses.
Nanoparticles made with snail slime aren’t just one size or shape. Yet for store-bought products, Cosma says, it’s important to closely control their form and function. For now, current snail-slime nanoparticles remain a mixed bag.
That’s may be due to snail slime containing lots of molecules. Several of them may play a role in changing Au3+ to Au0. Proteins are thought to be responsible. But snail slime also contains peptides, carbohydrates and more. Any of these may have the ability to help produce nanoparticles, says Salunke.
Jennifer Gubitosa is yet another chemist on the Bari team. She points out that things other than the slime recipe can “also affect the shape or morphology of the nanoparticles.” Among them, she notes, are temperature and pH. The good news: Such things “can be easily controlled.”
It’s even possible that the different types of nanoparticles in a jumble could be sorted out. Salunke suggests using a centrifuge (SEN-trih-fewj). It rotates a container of liquid fast. This creates pressure on its particles, pushing them outward. (You may have felt a similar pressure push you to the outside of your seat on a spinning carnival ride.) Smaller particles move through a liquid easier, collecting at the bottom. Bigger particles stay closer to the top.
Salunke suggests putting the nanoparticle mix into sugar water. It’s thicker than plain water. That improves the layering. Then centrifuge the mix “and in each layer,” he suspects, “you’ll get a particular size or shape.”







