High-tech solar ‘leaves’ create green fuels from the sun
Chemists harness the power of photosynthesis to make liquid alternatives to fossil fuels

Green plants take carbon dioxide, water and sunlight to create their own fuel. Scientists are making artificial plant leaves to do much the same thing in the lab. The difference? Their end product is a liquid alternative to fossil fuels.
Simon Gakhar/Moment/Getty Images Plus
By Laura Allen
This is another in our series of stories identifying new technologies and actions that can slow climate change, reduce its impacts or help communities cope with a rapidly changing world.
Green plants are amazing. Powered solely by the sun, they make their own food, or fuel. Researchers around the world have been striving to make fuels for human use in much the same way. A new device taps the power of sunlight to make a liquid fuel.
If it can be affordably scaled up, the device might help decarbonize societies around the world. That’s a needed step toward limiting climate change.
The leaves of most plants contain chlorophyll. In the presence of sunlight, this green pigment converts water and carbon dioxide (CO2) into sugars and oxygen.
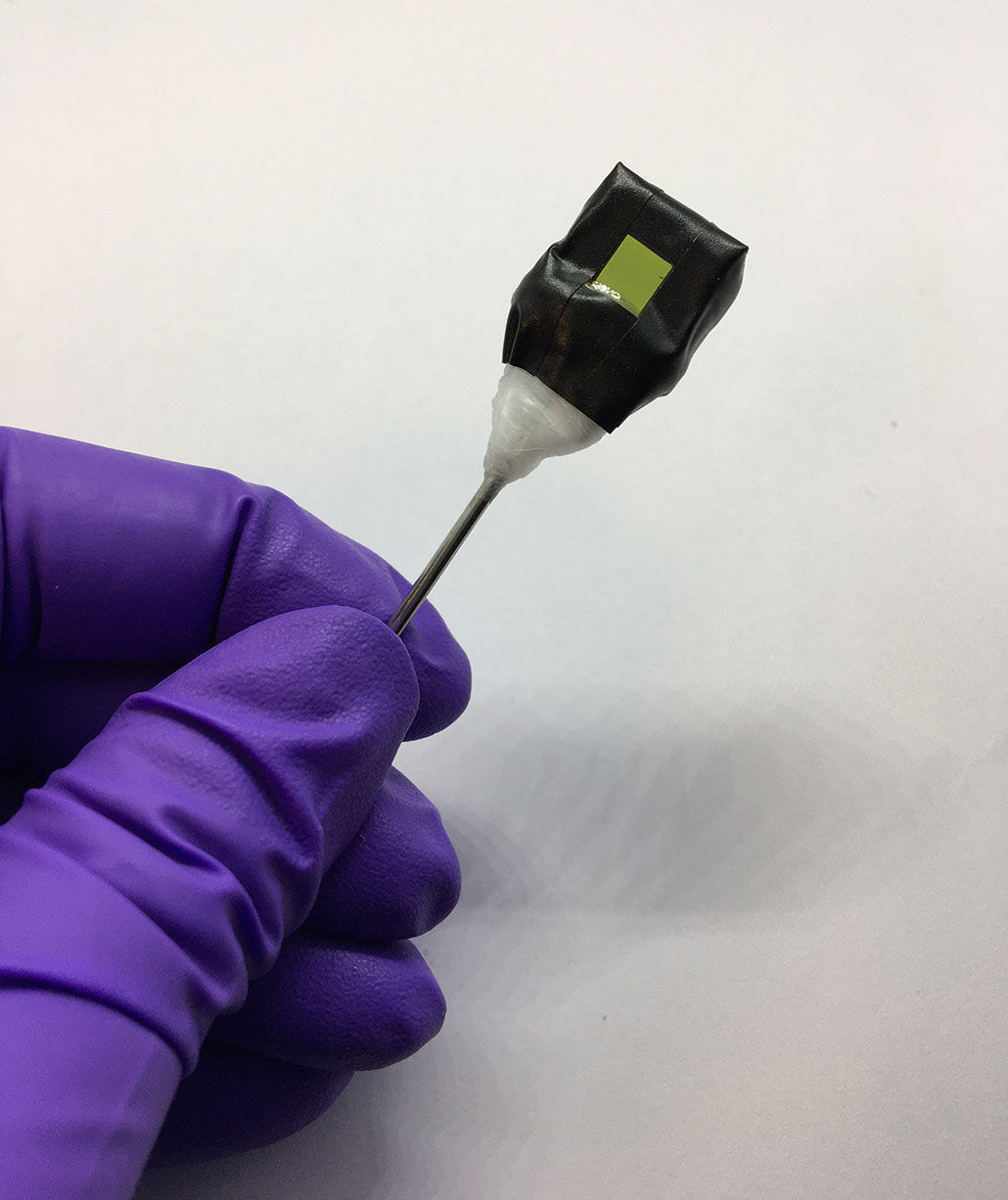
A single protein in chlorophyll splits apart molecules of water, explains Yulia Pushkar. She’s a biophysicist at Purdue University in West Lafayette, Ind. “In artificial photosynthesis,” she says, “we try to mimic the function of [plant] proteins.”
Breaking the bonds that link water’s hydrogen and oxygen atoms releases energy. That’s what powers the rest of photosynthesis. The end result: sugars that fuel a plant’s growth. Researchers want to make Earth-friendly replacements for fossil fuels. These might power heating or engines, such as those in cars and airplanes.
Researchers at the University of Cambridge, in England, have found one way to do this. Their new “artificial leaf” makes alcohol fuels — ethanol and propanol — from CO2 and water.
And it does this “using sunlight as the only energy source,” says Motiar Rahaman. He led the new work. Burning those alcohols will release energy, water and oxygen, this scientist notes — but no planet-warming gases.
Turning over a new ‘leaf’
Many different materials can split water molecules. The Cambridge team’s new device uses minerals for the task.
Their “leaf” consists of a solar cell made with a mineral, perovskite (Pur-OV-skyte). It absorbs light and releases electrons. This part is connected to a catalyst, made from copper and palladium. It converts CO2 into an alcohol fuel. Another mineral — bismuth vanadate — absorbs light and breaks water molecules apart, releasing oxygen.
Those elements all together make up a solar leaf.
In sunlit water enriched with CO2, this solar leaf begins the water-splitting process. This releases oxygen, and the catalyst converts the CO2 into alcohol fuels.

Rahaman’s group described its new process May 18 in Nature Energy.
Peidong Yang at the University of California, Berkeley also works on artificial leaves. This chemist praises the Cambridge “leaf” for being able to make liquid fuels from the sun. But there are more efficient ways to make solar fuels, he notes.
Instead of using light-absorbing minerals, Yang’s team works with bacteria. This method captures sunlight to split molecules of water into oxygen, electrons and protons. Bacteria then combine CO2 with these ingredients to efficiently make the chemical acetate (AA-seh-tayt). This small fatty acid can later be converted into other types of fuels.
Partnering with bacteria creates more than just fuel, says Joanna Kargul. She’s a biochemist at the University of Warsaw in Poland. There she runs its Solar Fuel Lab. “We can make plastics, fertilizers and basically everything we use that currently relies on fossil fuels,” she says of these bacterial systems.
Kargul, too, praises the Cambridge team’s device. But she notes that it’s challenging to take any of these devices beyond the lab and into the marketplace. The tools must first become far more efficient. And making them larger can run into new technical problems (such as short circuits).
Also, she says, relying on minerals is a less efficient pathway to create these fuels. Scaling up such systems will require lots of those materials. So it’s important they are readily available and not too costly.
Lanzatech, a company in Skokie, Ill., has just scaled up one artificial-leaf technology. Its system makes jet fuel, plastics and synthetic fabrics from bacteria fed with CO2 pollution from nearby factories.
When will solar-derived liquid fuels become widely available? Kargul thinks portable systems should be able to make such fuels, as needed, in another 25 years or so. These systems won’t rely on toxic products — or make pollution — while producing fuels straight from the sun.