Specially coated fabric could turn a shirt into a shield
Scientists create a coating for fabrics that captures and deactivates some chemical weapons

For kids and adults in war-torn countries such as here, in Syria, protection against chemical attacks can be a life-or-death need.
yeowatzup/Flickr (CC BY 2.0)
In April, bombs fell on Khan Shaykhun, a village in the Middle Eastern country of Syria. The explosions released toxic chemicals that killed or injured hundreds of people. Some toxic materials kill when people breathe them in. Others need only pass through the skin to be deadly. On battlefields and in war-torn countries such as Syria, protection against attacks by skin exposure to chemical weapons is a life-or-death need. And a new technology may help them. It would put such protection in a person’s clothing.
Scientists recently showed how to coat fabric with a light layer of a certain chemical mix. This coating can absorb and neutralize chemical weapons in minutes. The U.S. military wants such a fabric for soldiers. Civilians in war-torn regions also might benefit from clothing treated with the new technology.
Engineers described their new coating June 13 in Chemistry of Materials.
The coating’s secret ingredient is a chemical known as a metal-organic framework, or MOF. Such materials are composed of carbon-containing molecules attached to clusters of metal atoms. These materials have tiny holes, or pores, that can trap other chemicals. An MOF can interact with the trapped chemicals to break them down into harmless pieces. In the last few years, scientists have identified many different MOFs.
“This light [MOF] material can absorb the nasty chemicals or harmful agents, and at the same time deactivate them,” explains Omar Farha. He is a chemist at Northwestern University in Evanston, Ill. Farha did not work on the fabric coating. But he knows all about it. His research focuses on materials that can hold chemicals and break them into less toxic substances. Those materials include MOFs. In the past, his lab identified MOFs that could be used to store and break down toxic chemicals on clothing. (The researchers didn’t test these materials with chemical weapons. Instead, they used harmless chemicals with very similar structures.)
Story continues below image.
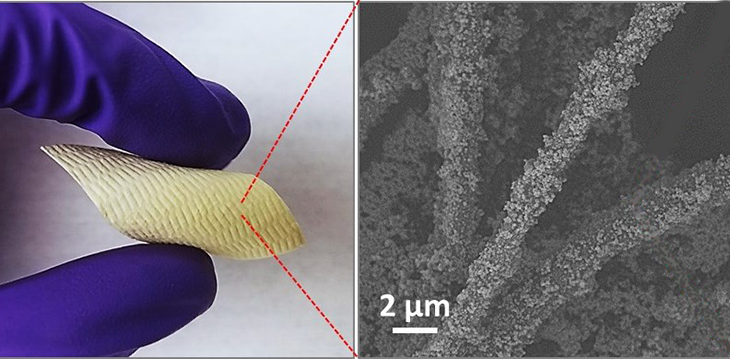
An un-sticky problem
After Farha’s team identified its candidate MOF, scientists looked for a way to use it as a chemical shield. That’s where Greg Parsons and his team came in. He’s a chemical engineer at North Carolina State University, in Raleigh. He also does research for the U.S. Army, which asked him to help add Farha’s MOF to fabrics.
This proved a major challenge. The MOF “doesn’t want to stick to fibers that are typically used in clothing,” Parsons found. The material is like a powder made of tiny crystals. If you tried to sprinkle it on a piece of clothing, it would blow right off. (Imagine pouring sugar or salt crystals onto your shirt — they won’t stay put!)
But Parsons had some ideas about how to solve that problem. He’s an expert in another area of science that requires getting materials to bond: electronics.
Many computer chips use a crystal made of silicon coated with thin layers of materials called metal oxides. To get metal oxide onto a chip surface, engineers use a process called atomic layer deposition. They expose the surface of a device to tiny quantities of chemical vapors, or gases. Those gases react with the surface. This chemical reaction leaves behind tiny amounts of a metal oxide. Over time, the process totally coats the surface with a very thin metal-oxide layer.
Parsons and his team used the same concept to solve their un-sticky problem. They first used atomic layer deposition to build up a thin layer of metal oxide on a fabric. That metal oxide let the MOF adhere tightly to the fabric.
Then they tested the treated fabric. They exposed it to a chemical similar to sarin. Sarin is a colorless, deadly poison that may be liquid, powder or gas. Experts believe Syrians were exposed to sarin gas during April’s attacks. A person can sicken or die by breathing or touching sarin. Treated clothing would keep sarin off someone’s skin.
Previous materials have been able to capture toxic chemicals. They did not, however, degrade them quickly. In lab tests, the new material broke down the sarin-like chemical in as little as five minutes. “The faster the better,” says Farha.
Parsons says the treated clothing worked in the lab, but it’s not ready for the real world just yet. He and his colleagues are still trying to find exactly the right MOF recipe to treat clothing. They also want to be able to treat lots of fabric at once. “Right now it’s a very slow and tedious process,” he says. “We’re thinking about how can we can do this quicker with the same good outcome.”
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.







