Spying on brains in action
Innovative tools let scientists observe brain activity of organisms on the go
Three new technologies could shed new light on what goes on inside our brains.
iLexx/istockphoto
You don’t usually stand still. Neither do most animals. Now three new tools let scientists see what’s happening inside the brain and the nervous system — even as an animal is moving.
It may sound like no big deal. Except it is. Living beings tend to be mobile. Getting a high resolution image of something small — say cells — tends to require that they don’t move. So that can present a major problem for researchers. And it’s that problem that the new techniques now attempt to conquer.
Each innovation builds on others. One is a new microscope that can film brain cells, or neurons, in small animals as they move or do other things. Another tool uses magnetic fields or radio waves to turn neurons on or off in living animals. The third technology offers a portable means to view what’s going on in the human brain.
These new tools can help scientists learn more about the living brain. And that’s always been a challenge. For starters, a skull covers the brain in many animals. That bone, like the brain tissue, is opaque. So you cannot see through them. And looking at pieces of them — removed from the body and under a normal microscope — doesn’t show what was happening in the live brain. Yet that brain activity is what so many scientists crave to better understand.
Fortunately, that’s what these new tools can showcase.
Your brain is the body’s command center. It sends and receives signals from all over. Those signals control what our bodies do and how we act. Studying these messages can shed light on how the brain normally interacts with the rest of the body. And that can better help scientists spot — and treat — what goes wrong in diseases that affect the brain.
A new kind of microscope
Elizabeth Hillman is a biomedical engineer at Columbia University in New York City. Her team’s new microscope can make movies of what goes on in the brains of animals — even those on the move.
Animals such as fruit flies, roundworms and zebrafish often serve as model organisms. They “stand in” for people in certain experiments. Yes, their brains tend to be quite small. They may have fewer than half a million neurons, compared to almost 100 billion in the human brain. Yet the nerve centers in these animals do many of the same things as the brains of people. And they often do it using the same types of cells, organs or signaling molecules.
To make it easier to study the brains of some animals, scientists have fiddled with their genes. Those genes now make certain proteins in their brain cells when those neurons fire. These cells now will fluoresce — briefly glow — when exposed to a certain wavelength of light. Some of these animals, such as roundworms and zebrafish, are naturally transparent. So when their proteins flash, this glow can be visible from outside the body. That means researchers don’t have to cut into the animals to see what’s going on inside them.
Some of these animals are small enough to fit under a microscope lens. In principle, then, scientists could attach a camera and take pictures of the activity in their brains.
There’s a catch, though. Most camera systems can’t take images of the whole brain quickly enough to see neurons in action. Catching the action means taking images at various stages of that activity. In the past, even the fastest 3-D image of a fly’s brain has typically taken at least one second. Notes Hillman: “That’s just not fast enough.” In people, signals to and from the brain can travel at more than 400 kilometers (250 miles) per hour. An action could start and finish long before a normal microscope’s camera captured even one image.
So she had been building microscopes that move a point of light around. Called SCAPE devices, these scan an object to make a 3-D image of it. But making pictures of one tiny point after another can be quite slow. She became curious about a different technique, called light sheet microscopy. It shines a plane of laser light into tissue, lighting many points at once. A camera focused on this plane takes a picture of the whole layer, including the flashes of any neurons that are firing.
Story continues below video.
The power of reflection
In theory, light sheet microscopy should be quicker than snapping images from spot to spot within a plane. However, to make a 3-D image, most people would move the sample up and down to scan each layer. Even in a more advanced system where the sample didn’t move, at least one camera lens would have to move to keep up with the sheet of light. However this occurred, the animal being studied would have to stay still. Tell that to a mouse or fruit fly.
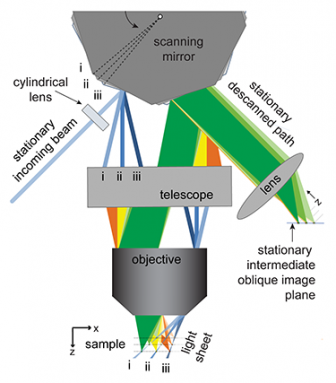
Then, Hillman recalls, “We had this crazy idea.” Instead of moving a sample or the lens, why not use a mirror to move the sheet of light? She and a graduate student brainstormed how they might do this.
Around this time, a mirror system came into their lab. This polygonal mirror was for another graduate student’s project. The wheel-like object had 12 sides — similar to the unit used in supermarket barcode scanners. Each side was a flat mirror. When this 12-sided mirror didn’t work for the other student’s project, Hillman started playing with it.
“I like playing with lasers,” she says. Hillman thought her team could use the angled mirrors to move laser light through a sample. All the while, they could keep the camera focused on the tissue being studied.
After lots of trial and error, her team got the new imaging system to work. “I can move the light sheet side to side,” says Hillman. Nothing else in the setup moves. But the small animal they’re looking at can be moving. All the while, her system can make a 3-D video of the brain and the activity of its neurons.
How it works
The group mounted the 12-sided mirror on a motor so it could move. Laser light shines at this moving mirror. The light bounces from there through a magnifying lens to the sample. That forms a diagonal sheet of light in the tissue.
Cells activated by the laser beam now fluoresce. That light enters the same lens and bounces off the mirror through some more lenses. Those lenses focus that reflected light into a fast camera where a computer records the image.
As the mirror moves, the sheets of light sweep through a sample. But the clever method for collecting and processing the fluorescent light means that the camera always stays focused on the lit-up plane of the sample. The computer then collects images and assembles them into a stack to create a complete 3-D picture. Put lots of pictures together — anywhere from about 20 to more than 100 per second — and you get a 3-D movie.
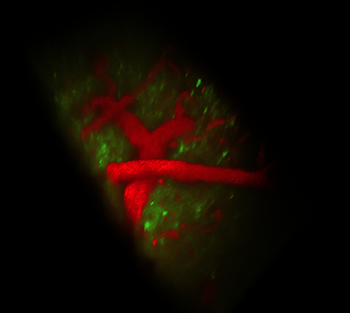
The 3-D scanner provides a new way to see the brain and nervous system in action. For example, Drosophila (Druh-SOF-ih-lah) larvae — “these yucky little maggots that fruit flies produce” — help scientists study ailments such as Lou Gehrig’s disease and spinal muscular atrophy, Hillman says. These diseases affect motor neurons — cells that help the brain control body movements. “With my system we can actually allow those organisms to crawl along.” And, she adds, “You can see the way the neurons in the brain are telling the body to move.” That, she says, is “helping us to figure out how [those neurons] don’t work in diseases.”
Scientists also are using the same method to study epilepsy in zebrafish. In people, that disease causes seizures. These are inappropriate surges of electrical activity in brain neurons. With the new method, researchers can image every neuron in the brain of a zebrafish to show just what happens during a seizure, Hillman reports. Applied to the fish’s heart, the method can show how the organ develops.
It has even been used in a nontransparent animal, a mouse, to see activity in part of its brain.
Hillman’s group has been working to use a single mirror instead of the 12-sided one from her earlier studies. That and other advances might boost the speed and quality of the 3-D videos she’s making. The group has also agreed to let a company (Leica Microsystems) develop the technology so it can be sold to other laboratories. That way, more researchers can use this tool for research on the brain.
Story continues below video.
New neural switches
But that method can’t explain everything happening in a living brain. For instance, suppose certain neurons fire when an animal starts moving. Do those neurons trigger everything that’s necessary for the motion? Or do they just trigger part of it? One good way to test the role of a firing neuron is to watch what changes when you switch it on and off, explains Sarah Stanley. She’s a doctor and neuroscientist at the Icahn School of Medicine at Mount Sinai (a health care system, in New York City). This approach, she explains, is “just like you switch on or switch off a machine to try and work out what it’s doing.”
One way to switch the cells on and off is through optogenetics. This technique uses light, or optics, to control what a cell’s genes do. That, in turn, can affect how neurons signal — communicate — with one another.
To make it work, researchers must first insert genes into certain neurons in a living animal’s brain. Those genes tell the cells to make unusual proteins. Those proteins act like one-way gates when they encounter certain wavelengths of light. The gates allow charged particles, called ions, to move either into or out of a neuron. If enough ions flow in, that causes an electrical buildup in the cell, and soon this neuron fires. This sends a chemical message across a tiny gap to a neighboring cell.
Once a lab animal’s neurons make the light-sensitive protein, researchers can use light to make the neurons fire or stop firing. To do this, researchers must first get light into an animal’s brain. So usually the animal is connected to a light source. “This can actually cause distress,” says Stanley. And that that might interfere with some behaviors a researcher might want to study, she notes.
She wanted to create switches for neurons that could work without needing a cable to bring light into the brain. Nanoparticles — bits of material just billionths of a meter in size — might solve the problem, she thought.
Stanley knew about a protein called ferritin. It can contain iron nanoparticles. “Metal nanoparticles interact with magnetic fields and radio waves to absorb energy,” she notes. Neither of those triggers needed a cable to deliver it.
Story continues below image.
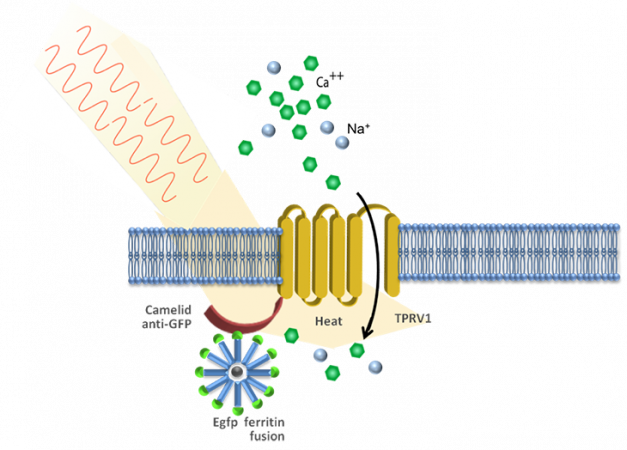
In earlier work, Stanley and other researchers had already injected ferritin nanoparticles into mice. They knew that once energized, nanoparticles could open ion channels so that ions could flow into or out of the cells. But injected nanoparticles only work near the spot they were injected — and they don’t remain there for very long.
Then Stanley’s team had an idea: Get cells to make their own nanoparticles.
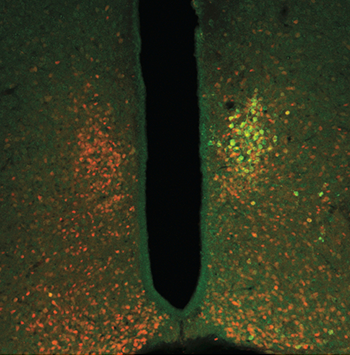
The team calls its new technology radiogenetics. The scientists use a harmless virus to change the genetic code in some cells. Afterward, these cells can make ferritin nanoparticles. Those particles also have bits of fluorescent proteins. That lets researchers see and photograph some of the neurons’ activity.
Stanley’s team is already using the method to study how the brain affects the body’s use of glucose, a simple sugar (sometimes called “blood sugar”). How efficiently the body moves this sugar from the bloodstream into cells can play a big role in diabetes and in medical conditions such as hypoglycemia (low blood sugar). The new method can turn on or off some neurons that sense or otherwise regulate sugar levels in the blood. That may also affect whether a mouse or other animal feels hungry and eats.
Stanley thinks radiogenetics will have lots more uses. Eventually the tool might even help treat diabetes or other diseases. “Most importantly, it can be used in freely moving and behaving animals,” she says.
A portable body scanner
Scientists want to see what happens in the brains of people, too. A PET scanner is one tool to do that. PET stands for positron emission tomography. This technology allows researchers to view such things as blood flow or oxygen use in any part of the body.
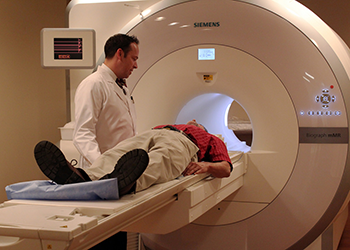
Before having a PET scan, a patient must drink or be injected with a chemical called a tracer. It’s a slightly radioactive material that is attached to some substance the body uses naturally, such as glucose. This sugar, for instance, fuels the activity in many cells. A tracer will now act just like its host substance, such as that glucose. And it will eventually build up in whatever part of the body uses the host substance most. For example, a tracer attached to glucose will build up in areas that are most in need of energy.
The tracer chemical gives off positively charged particles, called positrons. They crash into negatively charged electrons that are already in the body. Then pow! The two types of particles destroy each other. That gives off a type of radiation known as gamma rays. The PET scanner can detect those gamma rays. A computer maps where they were emitted and displays this as a picture of the body part being scanned.
Traditional PET scans require patients to lie very still for 30 minutes or longer in a big machine. That’s because of the giant, super-heavy tubes that detect the gamma rays, Those tubes stay still, so the patient has to, too. Move and the picture will become fuzzy.
Within the last 15 years, however, researchers have been working on smaller gamma-ray detectors. Some can now fit into your hand, says Julie Brefczynski-Lewis. She’s a brain researcher at West Virginia University in Morgantown. By 2011, her colleague Stan Majewski suggested that these smaller detectors might work in a portable PET scanner. Majewski is a physicist. He now works at the University of Virginia School of Medicine in Charlottesville.
Together, Brefczynski-Lewis, Majewski and their team made the idea work. They call their portable PET scanner AMPET. The “A” is for ambulatory, which refers to walking. And the “M” is for the micro-dose of radiation associated with these scans. The device consists of a helmet with a ring of detectors in precise spots. Cables connect the helmet to a computer.
“What’s different about our device is it moves with the head,” Brefczynski-Lewis says. Someone can walk on a treadmill or ride a stationary bike throughout a scan. A small computer tethered to the helmet could even slip into a backpack so that the person wearing the scanner could go almost anywhere and do almost anything.
Story continues below image.

Right now, the team’s device scans a section of the brain that might be found under a thick sweat band worn around the forehead. The device can produce clear images of areas there, deep inside the brain, Brefczynski-Lewis reports. And compared to regular PET scans, the radiation exposure is much lower. That makes the new device safer to use for studying how the brain works in healthy people.
“The next model will cover the whole brain from top to bottom,” she says.
The AMPET scanner could help scientists study movement disorders, such as Parkinson’s disease. Its images also could provide insights into mental disorders, such as schizophrenia. Brefczynski-Lewis also hopes to use the device to study how we interact with difficult people. “They could be gesturing and talking in a free and natural way,” she explains. As that happens, the helmet would show what’s going on in their brains.
Hillman, Stanley and Brefczynski-Lewis all presented their emerging brain-imaging technologies at the annual meeting of the American Association for the Advancement of Science, last February, in Boston. All three innovations got funding from the BRAIN Initiative. That’s a government program started four years ago to support new technologies for studying the brain.
Who knows what innovations these and other projects might lead to in the future? Perhaps ideas from your brain might help!