Staph infections? The nose knows how to fight them
Bacteria in some people’s noses may be able to knock out antibiotic-resistant infections
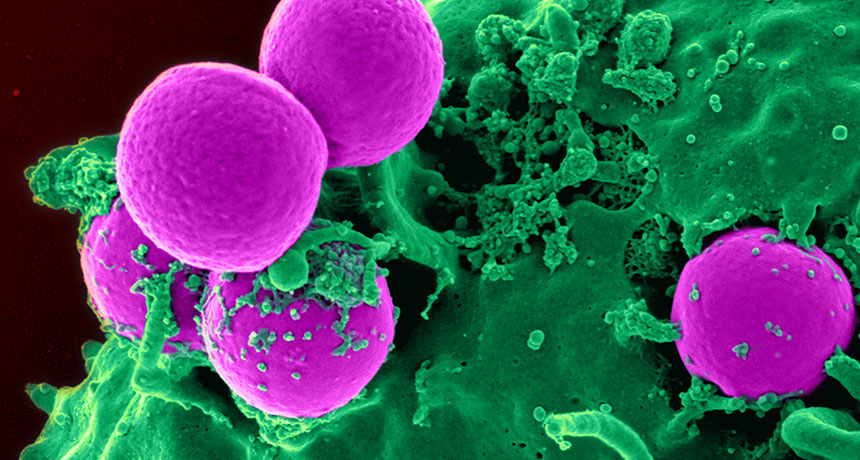
A compound made by bacteria that live in our noses may help fight infections of antibiotic-resistant MRSA germs (pink).
NIAID, NIH/WIKIMEDIA COMMONS
By Eva Emerson
MANCHESTER, England — The human nose isn’t exactly prime real estate for bacteria. It has limited space and food for microbes to eat. Yet more than 50 species of bacteria can live there. One of them is Staphylococcus aureus, best known simply as staph. This bug can cause serious skin, blood and heart infections. In hospitals, it can morph into a superbug called MRSA that’s extremely hard to treat. Now, scientists have found that the human nose can hold not only staph, but also its natural enemy.
That enemy is another germ. And it makes a compound that might one day be used as a new drug to fight MRSA.
“We didn’t expect to find this,” says Andreas Peschel. He studies bacteria at the University of Tübingen in Germany. “We were just trying to understand the ecology of the nose to understand how S. aureus causes problems.” Peschel spoke at a news briefing July 26, here, during the EuroScience Open Forum.
The human body is full of germs. Indeed, the body hosts more microbial hitchhikers than it does human cells. Many different species of germs live inside the nose. There, they battle each other for scarce resources. And they are experts at it. So studying nose bacteria might be a good way for scientists to search for new drugs, Peschel said. The molecules that microbes use to fight each other could become tools for medicine.
There’s huge variation in nasal microbes from one person to the next. For example, S. aureus lives in the noses of roughly 3 in every 10 people. The other 7 in 10 show no sign of it.
Trying to explain this difference led Peschel and his colleagues to study how microbial neighbors interact within the nose. They suspected that people who don’t carry staph might have other germy hitchhikers that block staph from growing.
To test that, the team collected liquids from people’s noses. In these samples, they found 90 different types, or strains, of Staphylococcus. One of these, S. lugdunensis, killed S. aureus when the two were grown together in a dish.
The next step was to figure out how S. lugdunensis did that. The researchers mutated the DNA of the killer germ to make many different versions of its genes. Eventually, they ended up with one mutated strain that no longer killed the bad staph. When they compared its genes to that of the killer strains, they found the difference. That unique DNA in the killer types made an antibiotic. It was one entirely new to science. The researchers named it lugdunin.
One of the most deadly forms of staph is known as MRSA (pronounced “MUR-suh”). Its initials are short for methicillin-resistant Staphylococcus aureus. It is a bacterium that normal antibiotics can’t kill. But lugdunin could. Many bacteria have evolved the ability to resist the germ-killing effects of one or more important antibiotics. So anything — like this new lugdunin — that can still knock those germs out becomes very attractive to medicine. Indeed, new studies show lugdunin also can kill a drug-resistant strain of Enterococcus bacteria.
The team then pitted S. lugdunensis against S. aureus germs in test tubes and in mice. Every time, the new bacterium defeated the bad staph germs.
When researchers sampled the noses of 187 hospital patients, they found that these two types of bacteria rarely lived together. S. aureus was present in 34.7 percent of people who did not carry S. lugdunensis. But only 5.9 percent of people with S. lugdunensis in their noses also had S. aureus.
Peschel’s group described these results July 28 in Nature.
Lugdunin cleared up a staph skin infection in mice. But it’s not clear how the compound works. It might damage the bad staph’s outer cell walls. If true, that means it might damage human cells too. And that could limit its use in people to a drug that is applied to skin, other researchers say.
Peschel and coauthor Bernhard Krismer also suggest that the bacterium itself might be a good probiotic. That’s a microbe that helps prevent new infections rather than fighting existing ones. They think doctors might be able to put S. lugdunensis in the noses of vulnerable hospital patients to keep staph infections away.
Kim Lewis studies antibiotics at Northeastern University in Boston, Mass. He agrees, in general, that studying microbes in the nose might help scientists find potential new drugs. Bacteria and other germs in and on the human body are collectively referred to as our microbiome (MY-kro-BY-ohm). But so far, Lewis says, scientists have found only a handful of potential new antibiotics by studying the human microbiome. (One of these is called lactocillin.)
Lewis thinks that lugdunin might be beneficial for use outside of the body. But it might not work as a drug that treats infections in the whole body. And these, he adds, are the kinds of antibiotics that doctors use most.







