Stashing more CO2 in the ocean could slow climate change
But changing the sea’s chemistry and biology to do this raises many environmental concerns

Changing the ocean’s chemistry or biology to pull more carbon dioxide (CO2) out of the air could help slow Earth’s rising fever.
Deb JJ Lee
This is another in our series of stories identifying new technologies and actions that can slow climate change, reduce its impacts or help communities cope with a rapidly changing world.
The ocean is Earth’s climate hero.
For decades, ocean waters have helped slow the rate of global warming. Indeed, over the past 250 years the seas have absorbed at least one-third of the carbon dioxide (CO2) emitted by human activities. This has kept that climate-warming gas out of the air.
But as CO2 emissions continue to rise, will the ocean be able to do even more?
Right now, time is running out to limit global warming by just cutting CO2 emissions. In 2015, 195 countries and other parties agreed to try to keep global warming below 2 degrees Celsius (3.6 degrees Fahrenheit). That’s in comparison to preindustrial times. This target would aim to avoid the worst impacts of climate change.
But over the next 75 years, Earth is on track to warm by about 3.2 degrees C. (5.8 degrees F.). That’s according to the Intergovernmental Panel on Climate Change, or IPCC. Even if all countries meet their current emissions-cutting pledge, the world would still warm during that time by some 2.7 degrees C. (4.9 degrees F.).
Slowing this rate of warming will require actively pulling CO2 back out of the air. This is called carbon-dioxide removal, or CDR.
CDR technologies are fairly new. Right now, they remove only about 2 billion metric tons of CO2 from the air each year. That’s less than 7.5 percent of the 37 billion tons of CO2 emitted each year by our use of fossil fuels (the drilling, transformation and burning of coal, oil and natural gas).
To meet the IPCC’s 2015 goal, each year through 2050 the world will need to remove 10 billion to 15 billion tons of CO2, says Gabriella Kitch. After that, you’d need to take out more each year. She’s a geochemist at the U.S. National Oceanic and Atmospheric Administration. Her office is in Silver Spring, Md.
Land-based CDR methods can get us part of the way there, she says. Those include planting trees and restoring coastal ecosystems. Some operations are also being built to directly capture CO2 from the air. In the end, all of these would take out only about 10 billion tons of CO2 each year, Kitch says.
“What about beyond that?” That’s where the ocean comes in. “The big advantage of the ocean is its capacity,” she says.
“The ocean,” Kitch says, “can store about 19 times the amount of carbon that can be stored on land.” Toward that end, researchers are seeking ways to tinker with its chemistry and biology.
There are a few basic strategies. Photosynthesis uses CO2. So we could try to grow more algae and other organisms that use photosynthesis. Or we could raise the pH of seawater (make it more alkaline) so that it can neutralize more of the acidic CO2. Or we could pull some CO2 out of the water so there’s room for it to take more out of the air.
But CDR in the open sea is largely untested. The ocean is vast and complex. Its conditions are also hard to monitor and change all the time.
And there are long-standing concerns about environmental impacts. Water changes or an overgrowth of algae might create ripple effects through ecosystems, critics note. They could pose risks to local wildlife — or even produce methane or other greenhouse gases. Right now, there are few data on such risks.
But time is the biggest challenge. Researchers are racing to explore our options before climate warming worsens.
A flood of money became available last fall to fund dozens of new ocean-CDR projects.
The snapshots below feature the six most-talked-about strategies. They point to what’s good and bad with each. They also note key questions that researchers must answer before society adopts any of these in a big way.

Ocean iron fertilization
Ocean phytoplankton are tiny photosynthetic algae. They live near the water’s surface.
Like land plants, they need sunlight, CO2 and nutrients (such as nitrate and phosphate) to live. To really thrive, though, they need smaller amounts of some nutrients — micronutrients — especially iron. But iron is in short supply in many parts of the ocean.

Observations in the 1980s noted that iron-rich dust blowing from continents to the ocean could lead to large blooms of phytoplankton. American ocean scientist John Martin proposed adding iron to the sea on purpose. He hoped it would kick-start algae blooms that would soak up more carbon.
Scientists tested this iron fertilization of the oceans 13 times between 1993 and 2009. Groups dumped iron sulfate into patches of the Pacific or Antarctic oceans. This did make the ocean bloom with phytoplankton. But the tests were too small and too brief to show the long-term effect of doing this.
Those tests also triggered a different bloom: environmental backlash. Critics worried that seeding the ocean might lead to blooms of toxic algae. Or it might make ocean dead zones if rotting phytoplankton pulled too much oxygen from the water. In 2008, an agreement from the United Nations called for a halt on iron fertilization until the effects were better understood.
Now, the climate crisis has led to renewed interest, says Ken Buesseler. He’s a marine chemist at Woods Hole Oceanographic Institution in Massachusetts. In 2022, he and some colleagues formed a new research group. Its goal is to explore ocean iron solutions and propose how best to study them. This time, social acceptance and citizen involvement must be core features of any project.
“We have to come up with a way to scale this up that would be acceptable and reproducible,” Buesseler says. That includes addressing environmental concerns.
Seaweed farming
Red, green and brown seaweed grow quickly. Some types shoot up by tens of centimeters (8 or more inches) per day. They fuel that growth through photosynthesis, which absorbs a lot of CO2 from the ocean. When the algae die, they sink to the depths of the sea. There, the carbon they’ve taken in may now cycle through deep-sea food webs. Or it can be buried in sediments for decades to centuries.

Farming seaweed — growing it on floating, offshore platforms — ramps up this natural process. Once the algae are fully grown, the platforms would be sunk into deep water. Natural seaweed populations worldwide take up around 170 million tons of carbon each year, one 2016 study showed. Farming seaweed could boost that to about 1 billion tons each year. That’s according to a 2022 report by the U.S. National Academies of Sciences, Engineering and Medicine.
What that number means: Seaweed alone can’t make a huge dent in getting rid of the world’s excess CO2. Plus, farmed algae might compete for nutrients with other species, such as phytoplankton, some studies suggest. And that could offset some potential climate gains.
Artificial upwelling and downwelling
Some of the world’s best fishing grounds are off the west coast of Peru. They’re a clue to ocean conditions good for growth. Earth’s rotation affects prevailing winds that blow along the shore there. The winds push nutrient-poor surface waters away from the shore. Cooler, nutrient-rich water surges up from the deep to replace it. The nutrients from this upwelling feed phytoplankton and the regional food web.
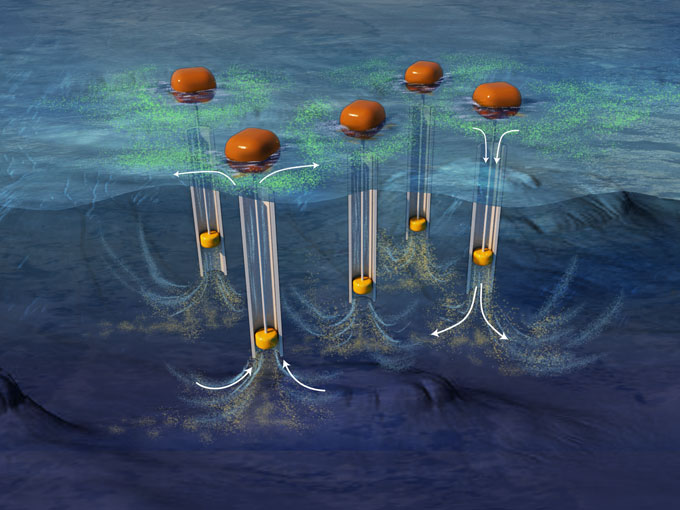
In theory, pumping nutrient-rich waters from the deep ocean toward the surface might mimic this upwelling in more places. Or carbon-rich surface waters might be sent downward to be trapped in the deep. That could be done through pumping or by making the water more dense — either by cooling it or raising its salt content.
Artificial upwelling also could be used to make seaweed farming or iron fertilization of the ocean more effective, computer models suggest. But there are a lot of unknowns: Deep water can contain a lot of CO2. If pumped toward the surface, it might escape into the air. Changing water flow also might harm sea life. And this pumping would take a lot of energy. If that comes from fossil fuels, this approach could actually add more CO2 to the air.
Enhanced rock weathering
The ocean gets its ability to take up CO2 from dissolved rocks. How? Minerals from eroded rocks on land wash into the sea over thousands to millions of years. They raise the pH of the seawater, making it more alkaline. Thanks to that alkalinity, CO2 gas in the air forms dissolved carbon-based carbonate molecules in seawater. Carbonate debris on the seafloor can hold carbon for as long as 100,000 years!

Making the ocean more alkaline could help it take in even more CO2. One way to do that is to dump in large amounts of finely ground alkaline minerals. This mimics the rapid weathering of rock. Researchers have done a few test projects in recent years. Results suggest this can neutralize ocean acidity and draw some CO2 out of the air.
But there’s not much real-world data yet on other impacts (such as any effects on sea life). A planned project in St. Ives Bay, England, faced local protests in September 2023. Protestors called for more study of possible effects on the bay’s wildlife, including the region’s prized gray seals. Some critics also point out environmental costs from mining and transporting minerals.
Electrochemical alkalinity enhancement
Another way to raise the ocean’s pH is to pull out acidic chemicals. One way might be to drive chemical reactions using electricity. Here, seawater would be pumped through an ocean-based system. The electricity would rearrange its water and salt molecules. They would split into two solutions, one acidic and one alkaline.
The alkaline solution would be mixed with seawater and returned to the ocean surface. This higher pH stuff would allow the water to pull more CO2 out of the air. The acidic share, meanwhile, might be neutralized or removed.
There are lots of questions about how this might affect the environment. Pumping large volumes of seawater could have big effects on marine life and local ecosystems. So could changing the water’s chemistry.
Existing systems for this method are expensive, too. They need a lot of infrastructure. And pumping the seawater and splitting the molecules takes a lot of electricity. That could produce more CO2 if it’s not from renewable energy sources. Combining a system with offshore wind turbines might help offset this.
Most work on this concept has been done in labs. But one pilot project was launched last August at Sequim Bay in Washington. It aims to capture 100 tons of CO2 per year.
Direct ocean capture
Electrochemistry could also be used to directly strip CO2 out of water. This is called direct capture. The CO2 that’s removed could then be buried in the deep ocean.
Ocean-based direct capture sends seawater through a large membrane. The membrane controls a chemical reaction between the water and another solution (such as sodium hydroxide). The reaction strips dissolved CO2 out of the water. It also raises the water’s pH so that it can absorb more CO2 from the air.

This system can be fully offshore. And in theory, it could be powered by wind or other types of renewable energy.
But one big disadvantage is its cost. It moves huge amounts of water through the facility. And its large membranes are costly.
Another worry is that drawing in large amounts of seawater could pose risks to marine life. And little is known about how changing the properties of the seawater might affect ecosystems.
Direct-ocean-capture technology has only recently moved out of the laboratory. In 2022 and 2023, the new company Captura did tests of it off the California coast. The company is now planning two small-scale projects this year in Canada and Norway.
The future of ocean CO2 removal
The best chance for success may come from trying several approaches, says Jessica Cross. She’s a biogeochemist who focuses on carbon in the environment. She works at Pacific Northwest National Laboratory in Seattle, Wash. One study last year suggested that using many methods might reduce the challenges linked with each.
A strong public pushback to some early field tests highlights “how challenging it’s going to be to do this kind of work,” Cross says. That’s why researchers are discussing how they might move forward on this tech while safeguarding the environment.
The ocean fills many roles for different people. “Emotions tend to run high when we’re talking about our coasts,” says Cross. So it will be essential to have communities on board every step of the way.








