Study acid-base chemistry with at-home volcanoes
Baking soda volcanoes are a fun demonstration, and with a few tweaks they can be an experiment, too

A few kitchen chemicals can give you an at home volcano. But you’re going to need more than one volcano for an experiment.
PeopleImages/E+/Getty Images
This article is one of a series of Experiments meant to teach students about how science is done, from generating a hypothesis and designing an experiment to analyzing the results with statistics. You can repeat the steps here and compare your results — or use this as inspiration to design your own experiment.
It’s a science fair staple: the baking soda volcano. This simple demonstration is easy to do. That clay mountain “smoking” in front of a poster board can be kind of sad, though. The whole thing looks like it was put together the morning of the fair.
But it’s not too difficult to turn this easy science demo into a science experiment. All that’s needed is a hypothesis to test — and more than one volcano.
A baking soda volcano’s foamy rush is the result of a chemical reaction between two solutions. One solution contains vinegar, dish soap, water and a little food coloring. The other is a mix of baking soda and water. Add the second solution to the first, stand back and watch what happens.
The reaction that occurs is an example of acid-base chemistry. Vinegar contains acetic acid. It has the chemical formula CH3COOH (or HC3H2O2). When mixed with water, acetic acid loses a positively charged ion (H+). The positively charged protons in the water make the solution acidic. White vinegar has a pH of about 2.5.
Baking soda is sodium bicarbonate. It has the chemical formula NaHCO3. It is a base, which means that when mixed with water, it loses a negatively charged hydroxide ion (OH-). It has a pH of about 8.
Acids and bases react together. The H+ from the acid and the OH- from the base come together to form water (H2O). In the case of vinegar and baking soda, this takes two steps. First the two molecules react together to form two other chemicals — sodium acetate and carbonic acid. The reaction looks like this:
NaHCO3 + HC2H3O2 → NaC2H3O2 + H2CO3
Carbonic acid is very unstable. It then breaks apart quickly into carbon dioxide and water.
H2CO3 → H2O + CO2
Carbon dioxide is a gas, which makes the water fizz like soda pop. If you add a little dish soap to your acid solution, the bubbles will catch in the soap. The reaction produces a big fwoosh of foam.
Acids and bases will react together until there are no excess H+ or OH- ions present. When all the ions of one type are all used up, the reaction is neutralized. This means that if you have a lot of vinegar, but very little baking soda (or vice versa), you’ll get a small volcano. Varying the ratio of ingredients can change the size of that reaction.
This leads to my hypothesis — a statement I can test. In this case, my hypothesis is that more baking soda will produce a larger explosion.
Blowing it up
To test this, I need to make volcanoes with different amounts of baking soda while the rest of the chemical reaction remains the same. The baking soda is my variable — the factor in the experiment that I am changing.
Here’s the recipe for a basic baking soda volcano:
- In a clean, empty 2-liter soda bottle, mix 100 milliliters (mL) of water, 400mL of white vinegar and 10mL of dish soap. Add a few drops of food coloring if you want to make your explosion a fun color.
- Place the bottle outside, on a sidewalk, driveway or porch. (Do not put it on grass. This reaction is safe, but it will kill the grass. I learned this the hard way.)
- Mix together half a cup of baking soda and half a cup of water. Pour the mix into the 2-liter bottle as quickly as you can and stand back!
(Safety note: It’s a good idea to wear gloves, sneakers and eye protection such as glasses or safety goggles for this experiment. Some of these ingredients can be uncomfortable on your skin, and you don’t want to get them in your eyes.)
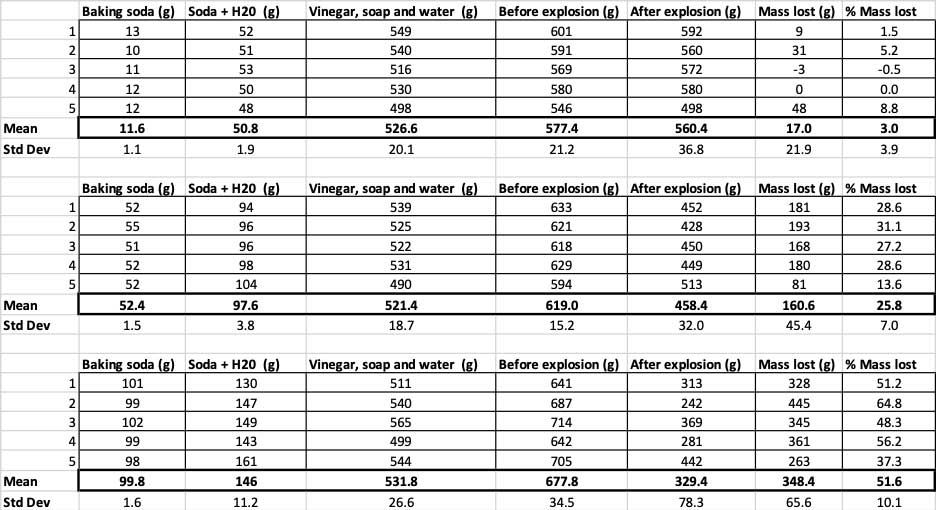
To turn this demonstration into an experiment, I’ll need to try this again, with three different amounts of baking soda. I started small — with just 10 mL, mixed with 40 mL of water. My middle dose was 50 mL of baking soda mixed with 50 mL of water. For my last amount, I used 100 mL of baking soda, mixed with about 50 mL of water. (Baking soda has a similar volume and mass, in that 10mL of baking soda weighs about 10 grams, and so on. This meant I could weigh the baking soda on a scale rather than have to measure it by volume.) I then made five volcanoes with each amount of baking soda, for a total of 15 volcanoes.
The explosion happens very quickly — too fast to mark its height accurately on a wall or yardstick. But once the eruption happens, the foam and water fall outside the bottle. By weighing the bottles before and after the reaction, and adding in the mass of the baking soda and water solution, I can calculate how much mass got ejected from each eruption. I could then compare the mass lost to show if more baking soda produced a larger explosion.

Using only 10 grams of baking soda, most volcanoes never made it out of the bottle. K.O. Myers/Particulatemedia.com 
Fifty grams of baking soda produced short jets of foamK.O. Myers/Particulatemedia.com 
A hundred grams of baking soda produced a tall whoosh of foam. K.O. Myers/Particulatemedia.com 
You don’t need to use a new 2-liter bottle each time. Just make sure you wash them out very thoroughly between volcanoes. K.O. Myers/Particulatemedia.com
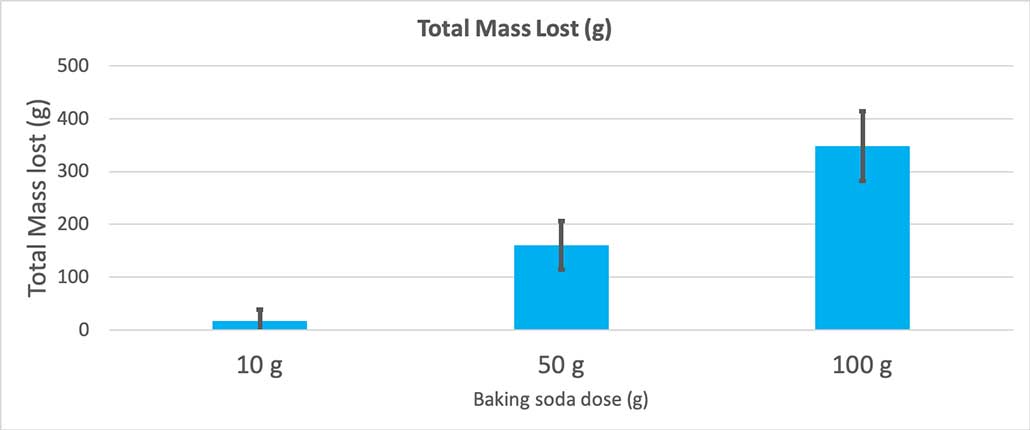
When I used only 10 grams of baking soda, the bottles lost 17 grams of mass on average. The eruptions were so small that most never made it out of the bottle. When I used 50 grams of baking soda, the bottles lost 160 grams of mass on average. And when I used 100 grams of baking soda, the bottles lost almost 350 grams of mass.
But that’s not quite the whole story. Because I added different amounts of baking soda and water to the bottles, there might not be as big of a difference here as I think. The extra mass from the 100-gram bottles, for instance, could just be because the reaction started out heavier.
To rule that out, I converted my numbers to the percent of mass lost. The 10-gram bottles lost only about three percent of their mass. The 50-gram bottles lost 25 percent of their mass, and the 100-gram bottles lost more than half of their mass.
To confirm that these results are different, I need to run statistics. These are tests that will help me interpret my results. For this, I have three different amounts of baking soda that I need to compare to each other. With a test called a one-way analysis of variance (or ANOVA), I can compare the means (in this case, the average) of three or more groups. There are calculators on the internet where you can plug in your data to do this. I used this one.
The test will give me a p value. This is a probability measure of how likely I would be to get a difference between these three groups as large as the one I have by chance alone. In general, scientists think of a p value of less than 0.05 (five percent probability) as statistically significant. When I compared my three baking soda amounts, my p value was less than 0.00001, or 0.001 percent. That’s a statistically significant difference that shows the amount of baking soda matters.
I also get an F ratio from this test. If this number is around one, it usually means that the variation between the groups is about what you would get by chance. An F ratio bigger than one, though, means the variation is more than you’d expect to see. My F ratio was 53, which is pretty good.
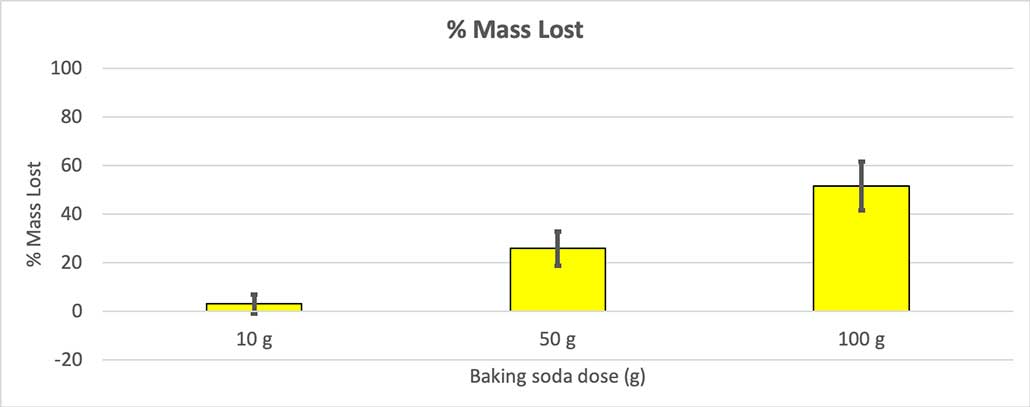
My hypothesis was that more baking soda will produce a larger explosion. The results here seem to agree with that.
Of course there are things that I could do differently next time. I could make sure that my bottle weights were all the same. I could use a high-speed camera to measure explosion height. Or I could try changing the vinegar instead of the baking soda.
I guess I’m just going to need to make more explosions.
Materials
- White vinegar (2 gallons) ($1.92)
- Food coloring: ($3.66)
- Nitrile or latex gloves ($4.24)
- Small digital scale ($11.85)
- Roll of paper towels ($0.98)
- Dish soap ($1.73)
- Glass beakers ($16.99)
- Baking soda (three boxes) ($0.46)
- Two-liter soda bottles (4) ($0.62)







