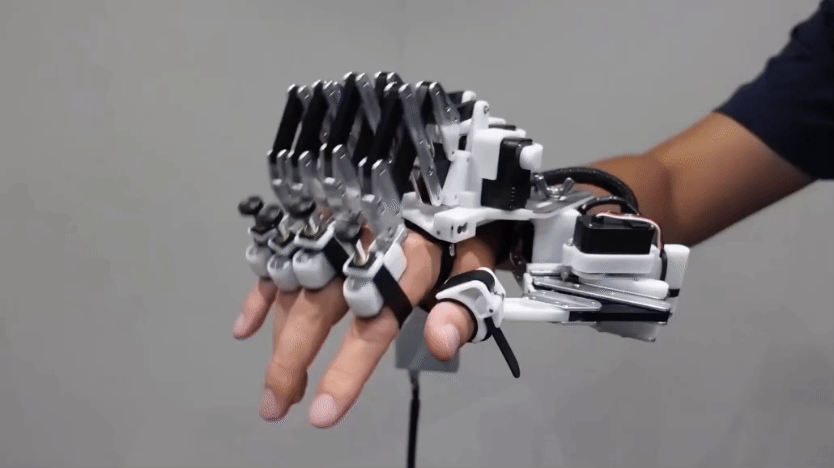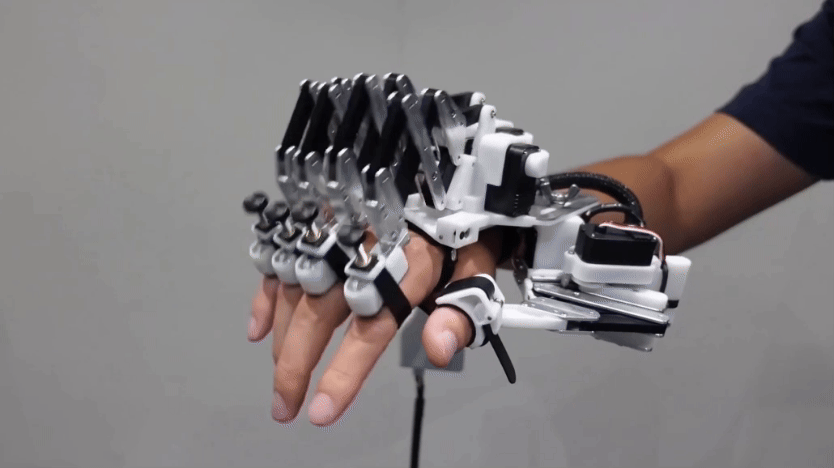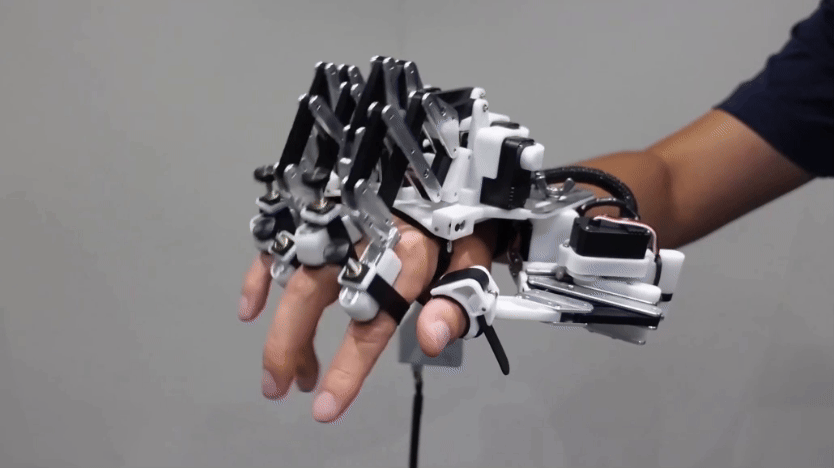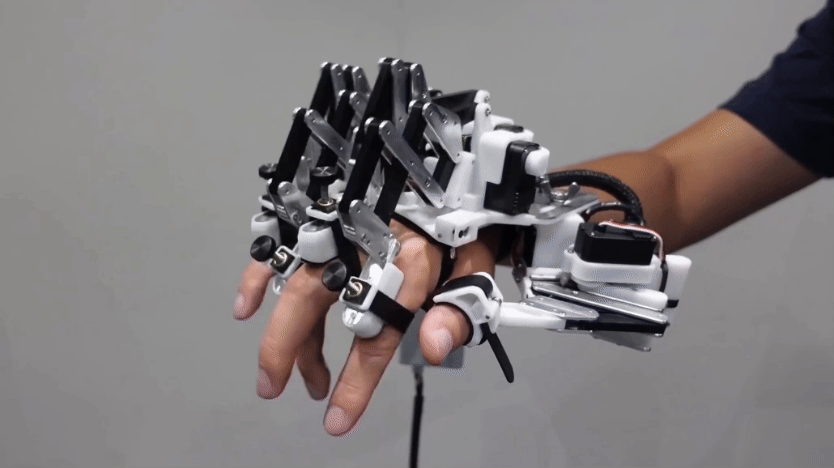
Bits of trees can make and store energy for us to use
This cellulose and lignin could lead to greener electronics

Within these trees’ wood are materials that could lead to cleaner, greener electronics.
Santiago Urquijo/Moment/Getty Images Plus
People tend to harvest trees for lumber and papermaking. But maybe electronics engineers are missing out on something here. “Turns out,” says Magnus Berggren, two major building blocks of wood have “a lot of electrical properties.” And he’s shown these blocks could serve as green replacements for some of the toxic materials used in electronics today.
Berggren is a physicist in Stockholm, Sweden. His team at Linköping University has been working to make parts for electronic devices from the forest. Right now, they’re focusing on two components of trees. One can generate energy. The other can store that energy, much as a battery does.
The first material is cellulose. Each molecule of this polymer consists of many sugar molecules, all linked into a chain. Cellulose helps put the crunch in lettuce. It makes up the fibers in your jeans and cotton T-shirts. And it’s “what you normally want to use for paper production,” says Berggren.
Lignin, the second material, is a structural component of plants. It’s what makes woody plants tough and rigid. Until recently, scientists thought lignin only did two things in green plants: transport water and provide structure. But a third role is starting to emerge, Berggren says. In its natural form, he says, this plant polymer “works like a battery.”
Cellulose also boasts an interesting electrical trait: piezoelectricity (Pee-AY-zo-ee-lek-TRIH-sih-tee). When squeezed, such materials release a zap of electricity (or flow of electrons). “Ever seen kids walking around with blinking shoes?” Berggren asks. If so, you’ve witnessed piezoelectricity at work.
Lignin can be tapped to store the electricity emitted in those zaps.
The power squeeze
Scientists have found many clever ways to use piezoelectric materials. For instance, some have used them to harness the energy in falling raindrops. But most piezoelectric materials contain harmful substances, such as lead.
Cellulose doesn’t, Berggren points out. His team has sandwiched bundles of cellulose nanofibers between two metal plates. Pressing those plates together squishes the cellulose. That triggers a zap! Electricity emerges. The researchers described their work in ACS Chemistry of Materials earlier this year.
Some device must then store the released electricity until it’s needed. Batteries can do this. But again, batteries aren’t very green. They contain harmful materials. That’s where lignin comes in. It can work as a battery-like alternative, says Berggren.
Lignin is a waste product of papermaking. Factories turn a tree’s cellulose into paper, then throw away its lignin. Berggren hopes to upcycle this waste.
Batteries cause electrons to flow from one place to another. That flow is known as an electrical current. In a battery, one material must give up electrons. Another must accept them. A typical lithium-ion battery uses lithium as the electron-donor. Graphite serves as the electron-acceptor.
Forest-born battery
Lignin can both accept and donate electrons, scientists reported last year. A weak acid encourages lignin to release electrons. Shuttle those electrons through a wire and — voila — you have electricity.
Berggren’s team used this trait to create its new lignin-based battery. Earlier this year, they described how it works in Advanced Sustainable Systems.
Typical batteries use a metal wire as a path to guide the flow of electrons into or out of the device. But since Berggren wants to make a battery-of-the-forest, he looked for a natural alternative to metal. Once again, he turned to cellulose.
This natural fiber can serve as a wire-like path for electrons. To make cellulose threads conduct electricity well, Berggren lined them with another chemical. It’s a polymer known as PEDOT. His team had used it before, in its early work on electronic plants.
PEDOT isn’t from a tree. But when it’s added to cellulose, those threadlike fibers can conduct electricity like a wire. And around that conductive cellulose, you can have a weak acid. Now you have a battery, he says. And two of its three materials come from the forest.
Berggren can imagine one day having cell-phone batteries made largely from forest products.
Tree-based electronics might also be useful in lighting, notes Michael Strano. He’s a chemical engineer at the Massachusetts Institute of Technology in Cambridge.
In general, Strano notes, we no longer sell products with toxic metals in them. But “lighting traditionally has always had these horrible materials,” he says. So they’ve been given “an exemption.” Cadmium and mercury are examples of such harmful ingredients. Governments allow the continued use of such toxic materials in lighting. (This situation is one reason his team is developing glowing plants. Such greenery provides lighting in a cleaner, safer way.)
One papermaking company in Finland, Stora Enso, is already putting its lignin waste to use, notes Berggren. They’ve produced “lignode” and “biographite.” These are lignin-based alternatives to graphite for use in new types of greener, more sustainable batteries.