Water sensor quickly detects algal poison
This cheaper, more sensitive test could be used to scout for evidence of harmful drinking water

Waves at Pelee Island in Lake Erie can turn bright green during a harmful algae bloom, but chemical testing is necessary to know whether the water is toxic to people.
T. Archer, National Oceanographic and Atmospheric Administration
Periodically, algae in lakes and streams will encounter a food bonanza. Within days, the growth of these one-celled organisms can mushroom into what scientists refer to as a bloom. If those algae make a toxin — a poison — this may make that water unfit to drink. The problem: The algae and their poison can be invisible to the human eye. But not to a new sensor. This new device can detect amazingly small quantities of the algal poison quickly and at low cost.
This means drinking-water officials may be able to warn people of risks before any signs of a sickening bloom become visible. They also can get to work treating the water to remove the poison before it reaches danger levels.
It’s a solution to a very real problem.
Not all algal blooms release toxins. But those that do can endanger human health. Indeed, such toxins made the drinking water supplies for one Ohio community too toxic to drink during part of 2013 and then for another city one year after that. Local officials told people to drink only bottled water for a few days. Events like these show why water-supply systems need to regularly test their water.

Today’s tests are not only expensive but also take hours to yield a result. The new low-cost sensor can cut that wait time down to just a few minutes. This device targets the same toxins — a group known as microcystins (My-kroh-SYS-tins) — that had poisoned the Ohio water supplies. One of the most potent of these poisons is microcystin-LR. It can cause rashes, headaches, diarrhea and liver damage. It some cases, it can kill people.
A guideline from the World Health Organization warns that people should not drink water containing more than 1 microgram of microcystin-LR per liter (µg/l). That’s one part per billion. U.S. Environmental Protection Agency guidelines recommend not drinking water with 1.6 µg/l or more of those poisons. Young children should get even less, EPA says — no more than 0.3 µg/l.
Yet higher levels do show up. Levels above 1 µg/l forced the city of Toledo, Ohio, to shut down its water supplies for two days in 2014. People in Carroll Township, Ohio, were told to avoid drinking their tap water for two days in 2013. The sooner cities can treat toxin-tainted water, the fewer people will face a risk of poisoning.
But the toxin doesn’t only show up in the Great Lakes or other sources of freshwater.
Raphael Kudela is a biologist at the University of California Santa Cruz. He and his colleagues recently found microcystin in ocean-dwelling mussels. They had collected these shellfish in California’s San Francisco Bay. The poison could have come from streams that flow into the bay.
Toxin levels in the mussels were lower than California’s suggested limits. Still, the state is in a major drought. If heavy rains were to occur, higher microcystin levels could enter the bay, the researchers say. Their new study on bay mussels tainted with the toxin appears in the November 2016 issue of Harmful Algae.
Wu Lu is an electrical engineer at Ohio State University in Columbus. “What you would like to have is testing in real time,” he says. By that he means engineers would like results in seconds to minutes, not hours. And that’s what his new sensor does. It can tell if microcystin is in liquids at levels as low as one part per trillion.
Lu and his graduate student, Paul Bertani, described their work on the new sensor at a meeting on September 15 in Toledo. It was hosted by Ohio Sea Grant and Ohio State University. (Sea Grant programs at universities in 33 states do research and outreach in cooperation with the National Oceanic and Atmospheric Administration.)
Teaming up
The new sensor took lots of teamwork. Jiyoung Lee, a microbiologist at Ohio State, is a co-author of the new research. She is among those scientists anxious to know more about how harmful algal blooms affect people’s health. She also wants to keep microcystin out of drinking water. “For toxin detection,” she notes, “sensitivity, accuracy and speed are the key.”
Lee knew of Lu’s work on biosensors. These are devices that use enzymes or antibodies to detect chemical residues left behind by living organisms. Lu’s sensors hunt for chemicals that are electrically charged (called ions) within some solution, such as water. As it happens, microcystin molecules in water do have a small positive charge.
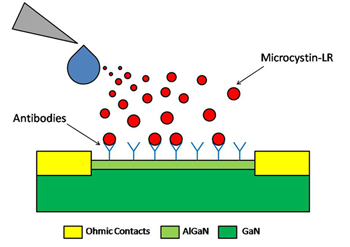
“I thought his biosensor would work for this toxin monitoring,” Lee says. Working together, her group and Lu’s created the new sensor.
It has two layers. You might think of it as a layered gelatin or a two-layered fudge. The bottom layer is gallium nitride. The top is aluminum gallium nitride. Both materials are semiconductors. That means they may, at times, relay an electrical current.
The sensor’s top layer is covered with antibodies. They bind any microcystin they encounter. So if a liquid flows over the sensor, its antibodies will latch onto the microcystins.
“In our tests, we used a 15-minute wait time,” says Bertani. Then the group put the sensor into a machine, which passes an electric current through the sensor. The machine measures the current in a process that’s a bit like the blood-sugar testers used by people with diabetes, Bertani explains.
When no microcystin is present, the current will flow at a certain low level. But if the water contains the toxin, the current increases. How much it rises will be a gauge of how much toxin was present.
Super sensitive
“The devices are highly sensitive,” Lu notes. They can detect as little as one part per trillion of microcystin in a liquid. Water-treatment plants could use the sensors to monitor levels of those toxins and how they change in response to weather or water treatment. The devices might even let researchers like Lee look for low levels of microcystins in someone’s blood. If it works, health officials could use the system to get a better idea of how algal blooms are linked to symptoms in people who drank tainted water.
Eventually, Lu’s team expects each sensor to cost only about $1 to $2. The machine to read those the sensors might cost another $1,000 or so. That equipment could make microcystin testing much easier (in part because the equipment would be portable).
The sensor “looks like it’s going to be an inexpensive, very rapid way to measure microcystin concentration,” says Glenn Lipscomb. He’s a chemical engineer at the University of Toledo who did not work on the devices.
One of Lipscomb’s projects tests filters that can be sold to help homeowners remove microcystin from tainted tap water. His team wants to test and compare how well those filters work in practice. The new sensor would make the chemical tests for his project quicker and cheaper, he says. Some people might even want to use such sensors to test their home’s water, he suspects.
The biosensor might also have been useful for Kudela’s study on shellfish in San Francisco Bay. His team used a method called LCMS. That stands for liquid chromatography (KRO-mah-TOG-rah-fee) mass spectrometry (Spek-TRAH-muh-tree). LCMS is “very accurate, but probably not as sensitive as the biosensor,” Kudela says. It also takes longer to get results.
If the test were cheap enough, commercial shellfish harvesters might use it to test local waters, he notes. High readings could provide early warning of a potential problem. The biosensor might be even more useful if it could be adapted to test shellfish tissue, Kudela says. But doing that would depend on how the tissue samples were prepared, said Young at Ohio State.
Lu’s team is now working to see how high a toxin level their sensor can measure. The engineers also are working to better calibrate those sensors. That means they’re looking to match a particular level of electrical conductivity with a particular concentration of toxin in the water.
So far, the Ohio State team has been testing the sensor in the lab. They know just how much microcystin is in the water in their tests. Out in lakes or water-treatment plants, however, other organisms and chemicals might confuse the sensor. The team will probably want to make sure those things don’t alter its sensor’s accuracy, Lipscomb says.
For now, he is hopeful, as is Lu, Lee and others. This project shows the value of researchers from different fields of science or engineering working together to solve a problem, says Lee. “It’s team science.”
Note: This story was changed on 11/7 to correct the definition for microgram per liter.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.