X-ray ‘eyes’
Movie directors cast tiny molecules in starring roles for their ultra-small-scale films

The photo Bullet Through Apple by Harold Edgerton captures the exact moment when a bullet pierces the fruit. To do so, his camera shutter had to be faster than the speeding bullet. For very small scale movies, far faster cameras are needed: ones that capture the action using X-rays.
Courtesy of Skinner Inc., www.skinnerinc.com
Look through a microscope at a drop of pond water. One-celled amoebas or other microbes will quickly swim into view. Tiny as they may be, these organisms are massive, hulking giants compared to the building blocks of their bodies — single atoms and groups of atoms bonded together, called molecules.
Atoms and molecules dance inside every part of our world — from squirrels and clouds to peanut butter and bicycle tires. Such atomic jiggles and jumps play a major role in the chemical reactions and other processes that make life possible.
Chemical reactions make or break the bonds that link atoms. Scientists would like to observe such reactions up close. To do that, they’re creating movies. Instead of chronicling the life of lions or break-dancers, these documentary film directors want to capture molecules in action. Their motion pictures could provide a very detailed picture of the chemistry of life.
 |
But catching this action isn’t easy. Molecules shuffle about too quickly to see with a microscope. Fortunately, a powerful laser — acting as a special type of flashlight — can help. It issues a beam of X-rays.
With it, scientists have recently begun recording a waltz danced by molecules inside plants and many algae. Filming on this project — Photosynthesis: The Movie! — will take several years. Although no one expects this flick to become a summer blockbuster, it could prove an instant hit among chemists and biologists.
The first challenge: good lighting
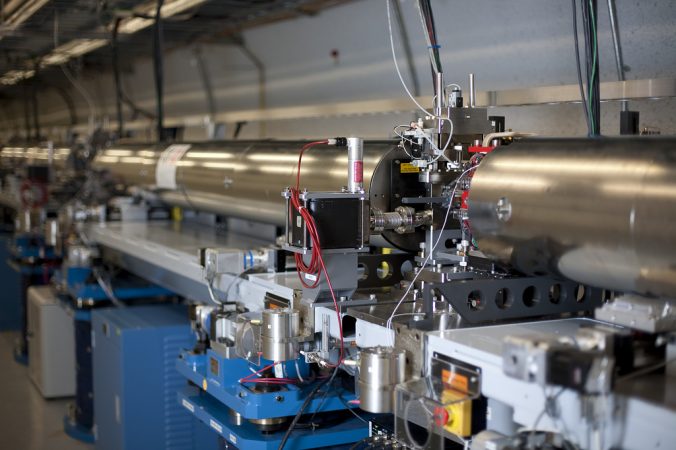
A beam of X-rays can bring atoms and molecules into focus only if it is bright enough. To help scientists make movies of atomic motion, an X-ray laser must be millions — if not billions — of times brighter than the X-ray machines that doctors use. The award for the brightest X-ray flashlight goes to a device called a free-electron laser.
 |
Why use X-rays?
Scientists tend to describe light not by its color, such as red, or by its type, such as visible. Instead, they describe its wavelength. The higher the energy of light, the shorter its wavelength.
Seeing something as tiny as an atom requires a light source with a wavelength that is smaller than the size of that atom. This rules out visible light. Its wavelengths are much too long. X-ray light, however, possesses the very, very short wavelengths needed.
Lasers can make the brightest, purest and tiniest spots of light possible. These devices line up light waves so they all travel together in the same direction. And the brighter the laser, the more light it packs into a small area. But packing a lot of small-wavelength light into a small area requires a very big laser. A national laboratory owned by the Department of Energy has built one of these giants at Stanford University in Palo Alto, Calif.
The device sits inside a 3.2-kilometer (2-mile) long underground tunnel. In a hall a little bigger than a football field, the laser’s 33 magnets — each about the size of a small car — help speed electrons along a wiggling path. (Electrons are very tiny particles that orbit around the nucleus, or center, of an atom.) The electrons’ jiggling motion produces X-rays.
Lenses focus these X-rays into a tight beam. Instruments along the beam’s path allow scientists to zap different materials with the laser. One of those instruments, an imaging machine, functions as a type of movie camera.
Explains Persis Drell, a Stanford physicist who until recently directed this facility, the free-electron laser “is allowing us to see the world in a way we’ve never, ever been able to see it before.”
Freeze and fry
Extreme brightness isn’t all that’s cool about this laser’s short-wavelength light. Its X-rays also emerge in incredibly quick flashes. Each lasts less than 100 femtoseconds, or quadrillionths of a second. By comparison, the blink of an eye can last one million times longer.
 |
The laser’s ultrafast pulses function much like the flash on a camera. When a photographer snaps a photo of a hovering hummingbird, the bird’s wings will almost always come out blurry. The reason: Those wings moved during the time the camera was capturing the picture. If the photographer uses a fast-enough flash to briefly illuminate the hummingbird, the camera will capture the bird as if frozen in mid-flap.
Like a hummingbird’s wings midflight, atoms and molecules are always moving. To see their dizzying dance as anything other than a blur, scientists need a flash that is as quick as the atoms.
A free-electron laser has the right stuff. Its 100-femtosecond bursts can freeze the action of even an atom. This ability has opened up a new frontier in science, Drell says. For the first time, scientists can see what the atoms are doing — and “on the timescales they’re doing it.”
In the process, however, this atomic-scale movie camera destroys the molecules that make up the film’s cast of characters.
 |
After snapping a hummingbird’s photo, the bird will fly away. Molecules pictured using a free-electron laser won’t be so lucky! The laser’s extremely bright X-rays fry them. Still, scientists can learn a lot before the molecules are obliterated.
The photograph at the top of the story shows a bullet piercing an apple. It is quite famous. Harold Edgerton at the Massachusetts Institute of Technology in Cambridge used a special kind of flash, called a strobe, to take this picture. In doing so, he froze the brief moment in time before the apple exploded. Scientists must freeze an even briefer period when photographing anything with a free-electron laser.
Experts call this technique “probe before destroy.” Physicist Henry Chapman pioneered the method. Because X-ray pulses are so incredibly fast, “you can get all the information out before the object really knows what’s happened to it,” says Chapman. He works at the Center for Free-Electron Laser Science in Hamburg, Germany.
Chapman was the first to use a free-electron laser to image the structure of a new protein. (Proteins are molecules that serve as a basic building block of life.) The journal Science called Chapman’s work one of the top research achievements of 2012.
Capturing one moment in time can be very important. But it’s when scientists string these moments together, one after the other, that things get really meaningful. These moments become a movie that maps the furiously fast dance of atoms and molecules during each step of a chemical reaction.
The secrets of photosynthesis
Photosynthesis is one reaction due to star in its own X-ray laser movie. In this reaction, a plant takes in carbon dioxide and water. Then proteins inside the plant follow a complex process to split the water molecules. Released atoms go into the creation of sugars that plants need in order to grow. Along the way, the process also generates oxygen as waste. (That’s good for us. People and other animals need that oxygen to breathe.)
Explainers
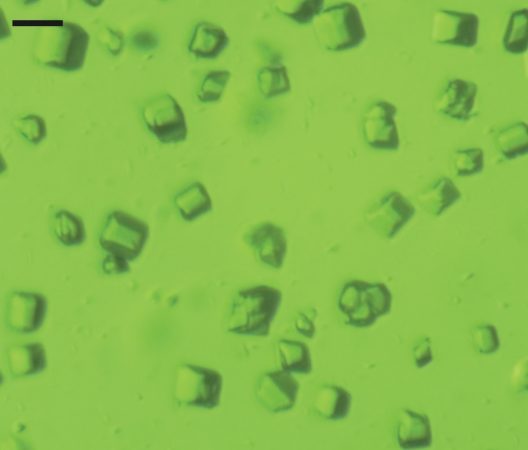
Several teams of scientists are hard at work seeking answers. Among them are Jan Kern and his coworkers at Lawrence Berkeley National Laboratory in Berkeley, Calif. They keep a glass tank full of bacteria in their lab. Just like plants, these microbes use photosynthesis to make food. Kern’s team is studying a certain protein inside the microbes. Called photosystem II, this protein breaks apart water during photosynthesis.
Kern’s group extracts this protein from its microbes and then turns it into crystals. Within each crystal, many, many protein molecules are arranged in a regular pattern. This pattern makes it easier to see how the molecules morph during a reaction.
“It’s not like a salt crystal which is solid and doesn’t really do anything inside,” explains Kern. His crystallized proteins can still perform photosynthesis. All they need is light, water and carbon dioxide.
Kern and his coworkers suspend their crystals in water and shine regular light on them. Then, they zap the crystals with the free-electron laser. The scientists know when each step of photosynthesis occurs. So they time the laser’s X-ray pulses to catch — and freeze — the action that accompanies each step.
So far, the team has uncovered details of the first two steps in the water-splitting process of photosynthesis. What comes next should be even more exciting, says chemist Junko Yano, who works with Kern.
 |
Once the experts have X-rayed all of the remaining steps, their motion picture will be ready for release. This ”filming” should take about two years to complete, but it is possible that free-electron lasers will not shoot the final scenes. Independently, other teams of X-ray moviemakers are also probing photosynthesis. Some of their “lighting” teams are using devices called synchrotrons. A synchrotron is a large, doughnut-shaped facility that uses magnets to speed up electrons until they produce beams of X-rays. There is “an interesting competition between the two technologies,” says John Helliwell. He’s a chemist at the University of Manchester in England who is not involved in the research.
Why is figuring out how photosynthesis works such a big deal? People have had a hard time re-creating in the lab what plants appear to do so effortlessly. For instance, many chemists have spent decades attempting to develop artificial leaves. The goal of that technology is to transform sunlight into a harvestable source of fuel, such as hydrogen.
So far, all approaches either use more energy than they produce or rely on materials that are costly or unstable. But an artificial leaf that efficiently produces hydrogen from just sunlight and water could help solve the world’s energy problems, says Kern. “Hydrogen can be used in fuel cells to drive cars or to heat your house,” he explains.
While rewarding, that’s not what motivates Kern to do this work. Instead, it is simple curiosity. Photosynthesis, he argues, “is a fascinating process.”
So much science, so few lasers
Free-electron lasers can uncover important details of some of the world’s tiniest, speediest and most important processes. Unfortunately, there aren’t many such X-ray lasers. Today, just two are up and running: the one at Stanford and another at the RIKEN Harima Institute in Japan. Each has space for only a few groups of researchers to work at one time.
The number of scientists who want to use these advanced tools far exceeds the space and time available at each. As a result, Drell says, “Not enough people can get experiments done.”
In 2015, scientists will fire up a new free-electron laser in Germany. It is expected to produce X-ray pulses 10 times faster than does the laser in California. Additional facilities are being built in Switzerland and Korea. “We can’t wait,” says Drell.
Nature still holds many secrets. More free-electron lasers could help reveal answers to many mysteries about the chemistry at play inside everything — including us.
Power Words
artificial leaf A device designed to efficiently harness sunlight to perform work. It is not a living system, as a true leaf is. It may be an electronic device or some other machine that derives much or all of its energy from the sun’s radiation.
atom The basic unit of a chemical element or any molecule. Atoms have a nucleus. That nucleus contains protons and neutrons and is surrounded by electrons.
chemical reaction A process that changes the way atoms are arranged within a substance.
crystallography The study of the arrangement of atoms inside a crystal.
electron A subatomic (meaning smaller than an atom) particle. Each electron has a negative charge and orbits around the nucleus of the atom to which it is bound.
enzyme A molecule that speeds up a chemical reaction, typically inside a living thing.
femtosecond One quadrillionth (1/1,000,000,000,000,000) of a second.
free-electron laser A light source that can emit a focused beam of radiation in a range of wavelengths, from microwaves to visible light or even X-rays.
fuel cell A device that converts chemical energy into electrical energy. The most common fuel is hydrogen, which emits only water vapor as a byproduct.
hydrogen The lightest element in the universe. As a gas, it is colorless, odorless and highly flammable. It’s an integral part of many fuels, fats and chemicals that make up living tissues.
laser A device that generates an intense beam of coherent light of a single color. Lasers are used in drilling and cutting, alignment and guidance, and in surgery.
molecule A group of two or more atoms held together with chemical bonds. This is the smallest unit of any complete chemical.
photon A particle representing the smallest possible amount of light or other electromagnetic radiation.
photosynthesis A natural process that plants and some bacteria use to convert light energy into chemical energy.
photosystem II (or photosystem 2) One of two proteins that contain chlorophyll and play a pivotal role in photosynthesis.
physicist A scientist who studies physics, or the interactions between matter and energy.
protein A molecule that contains carbon, hydrogen, oxygen, nitrogen and usually sulfur. Proteins form the basic building blocks of the cells in all living things.
shutter The mechanical, gatelike closure in a traditional camera that sits between the lens and any light sensitive material, such as film.
strobe A brief flash of light.
spectroscopy The study of light patterns that reveal the chemical elements making up a material.
synchrotron A large, doughnut-shaped facility that uses magnets to speed up particles to nearly the speed of light. At these speeds, the particles and magnets interact to emit radiation.
wavelength The distance between one peak, or crest, of a wave of light, heat or other energy and the next corresponding peak or crest.
X-ray A form of highly energetic radiation with a very short wavelength, ranging from 0.01 billionths to 10 billionths of a meter.








