Your DNA is an open book — but can’t yet be fully read
The stories your genes can tell are currently full of blank pages

There are many companies that offer to read your DNA, but they can’t fulfill all the promises you see in their ads.
cooperr007/iStockphoto
In Nevada, 40,000 people are stepping up to the cutting edge of medicine. They are getting their DNA deciphered by the testing company Helix. This is part of the Healthy Nevada project. The idea is to mix genetic and medical data with information about the environment. Doing that, researchers may get a clearer picture of all the things that can affect health. These free tests are going like hot cakes.
The project launched a similar partnership in 2016 with another testing company, 23andMe. Five thousand people living in northern Nevada were offered a free test kit for joining the program.
“Within 24 hours, 5,000 people had broken our website and signed up really enthusiastically,” says project head Joseph Grzymski. He’s a computational biologist who works at the Desert Research Institute in Reno, Nev. The project offered another 5,000 kits. “Within 24 hours that sold out,” Grzymski says. “We had 4,000 people on a waiting list.”

Even without an invitation or a free deal, people are flocking to these tests. Last year, more than 7 million people sent their DNA to testing companies.
DNA testing is now common, says Howard Hochhauser. He was the interim chief executive at Ancestry. This is an online genealogy company that helps people make family trees. The company also tests DNA to help people find relatives. “DNA testing is no longer a niche interest. It’s a mass consumer market, with millions of people wanting to experience the emotionally powerful, life-affirming discoveries that can come from simply spitting in a tube.” Hochhauser said that when talking about record-setting 2017 holiday sales at Ancestry.
I am the molecular biology writer at Science News. I also am one of the 7 million who wanted a read on my DNA. I thought it might help me learn about myself and my heritage. And I went all out.
My DNA is now part of the data banks of genetic-testing companies Ancestry, 23andMe, Family Tree DNA, Gencove, Genos, Helix, Living DNA and Veritas Genetics. Based on their tests, I learned some things about myself. I also learned about the glaring limits of today’s mail-order genetic tests.
Broad business
Each person’s DNA holds all of the instructions for building their body. Companies claim that they can read nearly everything about a person from that DNA. Some firms use DNA details to trace family trees. Some give diet advice based on your particular genes. Some also claim they can look at your DNA and tell you the best way to exercise to burn fat or to build muscle. Others go further out on a limb. They claim that testing a handful of genes can reveal a child’s future potential. Then there are kits that let two friends virtually mash up their DNA to see what it would look like if they had a baby. Some kits even claim to reveal superhero traits.
Some applications are clearly silly. But others are serious medical business. People can buy tests that examine versions of genes that have been linked to a higher risk for getting cancer, high cholesterol, diabetes, Alzheimer’s disease or Parkinson’s.

Human Longevity pairs a readout of a person’s DNA with extensive body imaging, blood tests and other medical screening. That lets the company gauge someone’s health with the goal of increasing lifespan. Scientists say it’s probably going to take this sort of all-inclusive information to really personalize medicine. However, few people can afford the $25,000 price tag.
These companies are part of a growing trend often called personalized, or precision, medicine. Health care systems are adding DNA data to medical records. They are hoping to better tailor treatments to individual patients or even to prevent illness.
Testing companies draw on databases compiled by research studies to make predictions about a customer’s health.
Some testing companies share their data with researchers who study human health and genetics. Some do their own studies. And some use the data to make money. They sell it to drug companies. I opted to allow the companies to share my data with researchers. (You don’t have to choose that option. But I like the idea of adding to science.)
More research is needed. Scientists still don’t totally understand how to apply the story in a person’s genetic instruction book — or genome — to health care. Every researcher I spoke with told me that such a goal is what they aspire to. It’s not something they expect to succeed at until sometime far in the future.
“There will be a time when many more common health conditions will have tests you can do that will really inform you,” says Bryce Mendelsohn. He’s a geneticist at the University of California, San Francisco, or UCSF. “But it will be years, years — decades — before we’re really at that stage.”
In August, Mendelsohn opened the Preventive Genomics Clinic at UCSF’s Benioff Children’s Hospital. There, he counsels adults on what type of genetic testing is right for them. He also tells them what they can expect from the results. He’s not against genetic testing by mail. However, he tells his patients, it should be for entertainment only.
“If they say, ‘I want to know about my ancestry. I want to know how much Neandertal I have,’ I say: ‘Great, that’s what consumer genetic testing is for.’”
But for important medical information, Mendelsohn orders tests from labs that are certified to make thorough diagnoses of health risks. He makes sure his patients know what to expect and what their results will mean.
“It’s not that companies that are selling tests are somehow evil,” he says. They just promise too much. “I just tell people up front, if you’re going to get this test, the odds are that you’re going to come back with nothing.”
False security
Daniel Cressman is a real estate broker in San Francisco. He wasn’t expecting any troubling or surprising results when he decided to have his DNA tested. He did it “just out of curiosity.” He wanted to be on the leading edge of what he sees as the wave of the future. One day soon, he predicts, “You’ll go to the doctor and the first thing they’ll do is pull up your genome.”
After hearing about the different levels of genetic testing that are being offered, Cressman decided he wanted the thorough approach — whole-genome sequencing. That is when a person’s full genetic instruction book is deciphered (not just certain parts of it). Cressman reviewed companies and settled on Veritas Genetics. The company will sequence a person’s genome for $999. Others offer whole genome sequencing, too. However, they can cost anywhere from $1,295 to more than $25,000.
“We tell people, ‘If you’re lucky, you have a boring genome. That’s what you really want.’ ”
— Gail Jarvik, Univ. of Washington
Some of Cressman’s family and friends are not on board with genetic testing, he says. “Some people ask, ‘What if you discover something you don’t want to know?’” But Cressman thinks it’s usually better to know what’s potentially in store. “If I’m flying San Francisco to New York on an Airbus and there’s a crack in the wing, I’d kind of like to know before I get on the plane.” He didn’t discover any cracks in his genes that would cause him or his doctor concern. “Nothing popped out at all,” he says.
Like Cressman’s, my DNA’s story, based on Veritas’ whole-genome sequencing, turned out to be pretty boring. For both of us, testing didn’t turn up anything in our DNA that is likely to cause us to develop a genetic disease. That’s good news, says Gail Jarvik. “We tell people, ‘If you’re lucky, you have a boring genome. That’s what you really want.’”
But just because Veritas didn’t find anything scary in our genomes doesn’t mean Cressman and I won’t develop health problems. Getting a clean bill of health based on your genes “can be very misleading — and falsely reassuring,” says Jarvik. She heads the division of clinical genetics at the University of Washington in Seattle. It also can be a bit of a letdown for some people.
“I think [buyers are] being overpromised that getting the genome sequenced will tell them lots of things that it won’t,” Jarvik says. “I’ve definitely run across people who have … been very disappointed because they didn’t learn as much as they thought.”
No drama
Most healthy people who send off their cheek swabs or saliva samples for DNA testing should expect to have a boring genome, says Leslie Biesecker. He’s a geneticist at the National Institutes of Health in Bethesda, Md. At this stage of the science, he says, genome sequencing is not useful for most people.
DNA sequencing does play a role in diagnosing some inherited diseases. It also can detect the mutations — gene changes — that can sometimes lead to cancer, Biesecker notes. But he doubts the value for most healthy people. There’s only a small chance, he says, “of finding something that would be useful to you medically.”
Only a very few people may have a rare genetic disorder and not know it, he says. “Occasionally we pick up some really severe stuff — hearing loss, cancer susceptibility or severe heart conditions — that you do need to do something about,” he says. But such powerful results are the exception.
Roughly 3 percent of healthy people might be such exceptions. That’s how many people might have known disease-causing variants in one or more out of 59 genes on a list. The American College of Medical Genetics and Genomics put together the list. The 3 percent statistic is from a 2015 report in Genetics in Medicine. Variants in those 59 genes are considered “medically actionable.” In other words, people who have an unusual form of one of those genes could take medicines or see their doctors more often for tests. Those actions might help catch cancers early, or lower someone’s risk of getting sick.
Scientists call genetic variants SNPs. It’s pronounced “snips” and stands for single nucleotide polymorphisms (NU-klee-oh-tyde Paa-lee-MOR-fizms). It’s a change in a single DNA letter, or nucleotide. Most SNPs are harmless. But sometimes these variants may lead to disease. Usually that happens when the variant causes a protein not to work correctly.
To get results on most of those medically important variants a person needs a doctor to sign off. Rules by the U.S. Food and Drug Administration, or FDA, require this. That’s why Veritas requires a doctor’s order before it will analyze someone’s DNA.
Last year, FDA opened the door a crack for one DNA-testing company, 23andMe. That company now can offer its customers information about diseases related to changes in certain genes. No prescription or referral is needed. For example, the company can now tell customers whether they have one of three variants in the BRCA1 and BRCA2 genes that raise a woman’s risk of breast cancer. (There are thousands of variants in those genes linked to breast cancer. The company, however, may only report on three.)
Carried away
People inherit two copies of most genes — one from mom, one from dad. Many of the variants on the list of 59 genes cause trouble when just a single copy is inherited from one parent. Geneticists refer to such genetic variants as “dominant.”
Some dominant variants lead to rare conditions. These include changes in the BRCA1 and BRCA2 genes that can cause breast and ovarian cancers. Other genetic variants on the list have been linked to certain heart problems, nerve disorders and other serious health issues.
Most people, including me, probably don’t have defects in single genes that could bring on a health crisis. What we pass on to our children, though, is another matter. Many people carry one copy of a “recessive” disease-causing SNP. Having one out of two copies of a gene with a recessive SNP doesn’t usually cause trouble. That’s because the healthy copy can cover for the faulty one. But people could pass the recessive variant on to their children. A child who inherits this recessive, disease-causing SNP from both parents could become very sick. That’s the case for conditions such as cystic fibrosis and Tay-Sachs.
23andMe, Veritas and other companies offering genetic test kits can now give information about a person’s “carrier status.” They identify whether you have a copy of a recessive-disease SNP that your kids might inherit.
Early studies suggest that, on average, most people are carriers for two recessive genetic diseases. Some carry no such variants. Others have up to seven or eight. I’m average. I carry two, or three, depending on the company doing the test. (I didn’t get the same results from every company.)
Being a carrier for a life-threatening genetic disease could influence someone’s decisions about having children. When both members of a couple carry disease-causing SNPs for the same gene, the couple has a 25 percent chance of having a child with the disease. Some couples might risk it. Others might decide not to have children. Some might get help from fertility clinics. There, doctors can perform in vitro fertilization. That lets them screen out embryos that have inherited two copies of the disease-causing variant.
Reality check
The vast majority of common traits, diseases and conditions result not from changes in single genes but from tweaks in many genes that work together. Such conditions are called polygenic traits. Researchers are not very good yet at figuring out how all these SNPs come together to affect appearance, behaviors and health.
Take type 2 diabetes. Hundreds of gene variants have been linked to this common disease. But none of those ensures a person will develop the disorder. Most SNPs have only a tiny influence on the chance a person will get a disease. Lifestyle and environment play a role, too.
And what should a person do about a variant that nudges up diabetes risk? Biesecker notes that your doctor will give you the same advice whether you have the variant or you don’t. “You can improve your health and longevity by improving your diet and exercising.” If you get the same answer either way, there’s little value in knowing whether you have the variant, he says.
Companies such as 23andMe used to tell consumers their risk of developing polygenic diseases. No more. In 2013, FDA banned the company from giving U.S. customers that information. Veritas is allowed to give medical information. But it doesn’t report risk of diseases risks that are affected by multiple genes.
Veritas and other companies are allowed to tell customers about polygenic traits that aren’t disease-related. 23andMe, for instance, examines 32 variants to predict whether your earlobes hang free or cling to the side of your head. And as many as 49 different spots in the genome could be involved in earlobe shape. This kind of information is just for fun. It is not medically important. But it is kind of cool to learn the genetics behind your ear shape.
Plenty of companies claim they can read your genes to tell you what you should eat or how you should exercise. Basing your diet on science seems like a smart thing to do. But Christopher Gardner says no. “Not today.”
Gardner should know. He’s director of nutrition studies at Stanford University’s Prevention Research Center in Palo Alto, Calif. He and his colleagues tested whether variants in three genes could predict whether someone would be more successful at losing weight on a low-fat diet or a low-carb diet. Those three genes are involved in how the body uses fat and carbohydrates. The idea was based on his team’s previous study of 130 people. People whose genetic profiles matched the diet they were assigned to lost three times as much weight as did those whose diets and genetics did not match.
Story continues below graph.
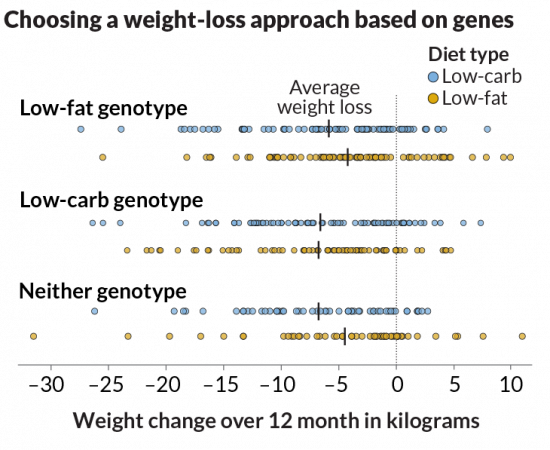
“It was plausible,” Gardner says. So the team expanded the study to more than 600 overweight and obese people. They assigned each person, at random, to either a low-fat or a low-carb diet. After one year, the researchers measured their weight to see whether a diet based on an individual’s genetics helped.
“The punch line is really short,” Gardner says. “It didn’t work.”
On average, participants in both diet groups lost about 5 or 6 kilograms (12 to 13 pounds) over the year. Some lost much more. Some lost less. Some even gained weight. But what happened didn’t matter whether the participant’s diet and gene profiles matched. Gardner and his colleagues reported their findings February 20 in JAMA.
Obesity is a complex trait. Studies have linked more than 200 variants to body weight. However, none tell the whole story of why people’s weight varies so much. “It isn’t that there’s one answer and in 10 years [we’ll have it],” Gardner says. “The answers will get progressively clearer and clearer. We could have a couple next year. They just won’t be the end-all, be-all answers.”
Drug reactions
If genetic testing can’t tell you much about health, diet and exercise, what is it good for? Even the most skeptical researchers I talked with said that one area holds promise: pharmacogenetics (FAR-mah-koh-gen-EH-tiks). This field of research explores how variants in DNA affect how people will react to certain drugs.
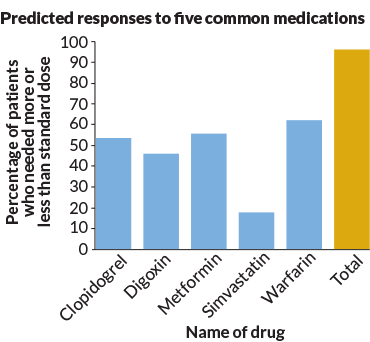
David Bick is a geneticist at the HudsonAlpha Institute for Biotechnology. It’s located in Huntsville, Ala. He sequenced the genomes of 45 people as part of a new program. Most of these people were white, highly educated and middle-aged. They ranged in age from 31 to 89 and paid about $7,000 for thorough physical exams to go along with the DNA testing and analysis. HudsonAlpha works with Huntsville-based Kailos Genetics to create profiles of how people might react to certain drugs. Such profiles might one day prove very useful, Bick says.
For example, doctors often prescribe a drug to people who have survived a heart attack. It goes by the name of clopidogrel. It’s often sold under the brand name Plavix. This drug keeps blood clots from forming. That cuts the risk of a second heart attack. For the drug to work, the body must use several enzymes to convert it into an active form. One of the enzymes is CYP2C19. “If you have a change in that CYP2C19 gene, you can’t convert [Plavix] to the active form,” Bick says. So if you have a heart attack and your doctor knows you have that variant, you could get prescribed a drug that won’t rely on that enzyme, he explains.
We all have about an 80 percent chance of inheriting a variant in at least one of the 806 genes known to be capable of altering how the top 100 U.S. prescribed drugs works. Researchers reported this December 22 in Genome Medicine.
Based on my Veritas report, I have at least 48 variants that can influence my reaction to dozens of drugs. I only take one of those drugs. So the information isn’t too useful right now.
Don’t dismiss genetic testing out of hand, though, says Robert Green. He’s a geneticist who has been studying people’s responses to genetic information for more than two decades. He works at Brigham and Women’s Hospital in Boston, Mass. Scientists will soon have a better handle on genetic information, he suspects. “What we see today is not the endgame. It’s not even the second inning.”







