The science of getting away with murder
A teen studies which cleaners work best at getting out blood

On TV shows, blood can leave a tell-tale trace. A teen tested different cleaners to see which one might truly erase evidence of a crime.
mosaic36/Flickr (CC BY 2.0)
PITTSBURGH, Pa. — Forensics-heavy shows such as NCIS, Bones and CSI: Crime Scene Investigation show science and technology solving seemingly impossible crimes. One regular viewer of such shows decided to take her fandom to the next level — the lab. Brynn Myers did her own forensics tests to find which popular cleaner can truly get rid of dried blood. The Missouri 16-year-old showed that when you need to clean up the evidence, bleach and laundry detergent are not your best bets.
The Oak Ridge High School sophomore presented her often-blood-stained findings here at the Intel International Science and Engineering Fair. Created by Society for Science & the Public and sponsored by Intel, the competition brought together some 1700 students from more than 70 countries to present their research findings. (SSP also publishes Science News for Students and this blog).
“When the science fair came up, I dreaded it,” Brynn admits. Her middle school science fair project hadn’t gone well. But when her science teachers found out about her love for NCIS and Criminal Minds, one of them gave her a book on forensics. The teen decided to study how scientists might detect blood if someone had already worked hard to remove the evidence.
She focused on three common household cleaners: bleach, laundry detergent and an oxygen-boosting cleanser.
Chlorine bleach is used to remove deep-seated stains and to disinfect. Most laundry detergents contain surfactants — chemicals that make it easier for substances such as the dirt on your clothes to dissolve into the wash water. Oxygen cleaners contain chemicals such as hydrogen peroxide, which also can disinfect surfaces.
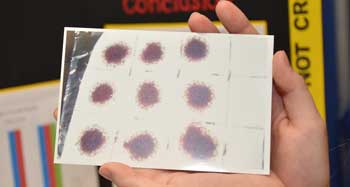
The teen washed each cloth using the same treatment up to three times. She examined the stains after each wash to gauge how much the blood had faded.
When it comes to visible stains, the oxygen cleaner worked best. It took only a single wash to remove all visual signs of blood from the cotton washcloths. The bleach batch was still stained after the first and second wash, but came out clean after a third run. Laundry detergent left a pile of blood-stained cloths — even after three washes.
But eyeballing surface stains is hardly the only way forensic teams probe for blood. Brynn took cloths that had been washed once, twice or three times and ran seven different forensic tests on them. Each test was designed to detect the presence of blood. Most of those chemical tests turned up evidence of blood, even when the visible stains were gone. Five of these tests failed to find any blood after washing with the oxygen cleaner, although another two types did find traces of the now-invisible blood.
If you’re at the scene of a crime and no blood is present, Brynn recommends looking to see if there was oxygen cleaner on the scene. Her data also show why running more than one test for blood is a good idea. Evidence that one technique misses might turn up by using another.
With her newfound knowledge, is Brynn contemplating a career in forensics? “It might be very interesting.” But still, she notes, “I am a little squeamish.” While she’s just fine with a little blood, the dead bodies are still a bit too much.
Follow Eureka! Lab on Twitter
Power Words
(for more about Power Words, click here)
bleach A dilute form of the liquid, sodium hypochlorite, that is used around the home to lighten and brighten fabrics, to remove stains or to kill germs. Or it can mean to lighten something permanently, such as: Being in constant sunlight bleached most of the rich coloring out of the window drapes.
chlorine A chemical element with the scientific symbol Cl. It is sometimes used to clean water. Compounds that contain chlorine are called chlorides.
disinfect To clean an area by killing dangerous infectious organisms, such as bacteria.
hydrogen peroxide A molecule made of two hydrogen and two oxygen atoms. Highly reactive, it can kill many tiny organisms, including germs.
peroxide A group of chemicals that contain a “bivalent” pair of oxygen atoms. Each oxygen atom has an unpaired electron orbiting it that is available to form bonds (attachments) with other atoms. Peroxides are oxidizing agents, meaning that they can react vigorously at room temperatures. Some are used as bleaches.
surfactant A chemical that decreases the attraction between water molecules. Manufacturers use such compounds to make it easier for water to spread on surfaces and to mix with other substances (such as oil).
Society for Science and the Public (or SSP) A nonprofit organization created in 1921 and based in Washington, D.C. Since its founding, SSP has been not only promoting public engagement in scientific research but also the public understanding of science. It created and continues to run three renowned science competitions, including the Intel International Science and Engineering Fair (initially launched in 1950). SSP also publishes this blog and award-winning journalism in Science News (launched in 1922) and Science News for Students (created in 2003).







