Sea changes
Carbon dioxide is making the oceans more acidic
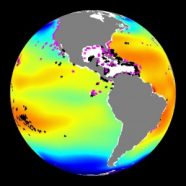
Every day, the ocean absorbs about 22 million tons of carbon dioxide. That’s about the weight of 15 million hybrid automobiles. Just as you can’t see the carbon dioxide that comes out of your own body each time you exhale, you can’t see the gas as it dissolves into the seas.
Space isn’t an issue. After all, the oceans cover 72 percent of the planet. Still, there is a problem brewing beneath the waves. Carbon dioxide in the air helps insulate our planet and keep it warm. But there can be too much of a good thing: In the last 200 years, humans have added a lot of extra carbon dioxide to the atmosphere by burning fossil fuels like coal, oil and gas to produce energy. Two hundred years’ worth of extra carbon dioxide in the atmosphere has bulked up our carbon dioxide blanket. Now, average temperatures around the world are rising. Scientists refer to this as global warming.
But effects of this global-warming gas go beyond the air and land. Much of the carbon dioxide emitted into Earth’s atmosphere ends up in the seas. Of every 10 tons of the gas added to the atmosphere, two or three end up in the water. The growing amounts of carbon dioxide that human activities add to the air have begun changing the chemistry of the oceans. It’s making them more acidic. This process is called ocean acidification.
Acids include liquids like vinegar and lemon juice that taste sour. These materials react with bases ― substances, such as ammonia or baking soda, that feel slippery — to form salts. Water is neutral, which means it’s neither an acid nor a base. Scientists measure acidity using the pH scale; acids have a pH between 0 and 7, and bases between 7 and 14. (Neutral water has a pH of 7.0.)
Ocean water is slightly basic, with a pH of about 8.1. But that number is changing. As the amount of carbon dioxide in ocean water goes up, the pH of ocean water goes down. And that means it becomes more acidic.
It’s happening now, and it’s happening fast. By the year 2100, if we continue to add the same amount of carbon dioxide into the atmosphere that we are adding now, the oceans will be more than twice as acidic as they were before the Industrial Revolution. The Industrial Revolution began more than 200 years ago. (That period describes the rapid growth of industry in the Northerm Hemisphere.)
Carbon dioxide is a gas. So its molecules are spread out and can flow freely within a space. But when they get into the ocean, the molecules break apart. Now their parts get together with other molecules in the water to form new molecules. These carbon dioxide molecules can become part of new gases, liquids or solids.
It’s hard to imagine that such a small molecule as carbon dioxide has the potential to drastically change the ocean, says Peter Neill. He directs the World Ocean Observatory in Boothbay Harbor, Maine. Molecule by molecule, the effect of extra carbon dioxide is harmless. But on the grand scale taking place today, the effect is substantial and chaning rapidly.
“We don’t really give it much thought,” Neill says, “but [ocean acidification] may be one of the most important aspects of what’s going on [with climate change]. And it may be the one that lasts the longest.”
The sea butterfly’s shell

You probably won’t notice changes to the water’s chemistry with a trip to the beach and a dip in the ocean ― even wearing your snorkel mask. But sea buttterflies will. They’re small (hardly 1 centimeter long), strange organisms. They can live in some of the coldest waters on Earth, near the North Pole and off the coast of Antarctica. Scientists suspect that as the oceans become more acidic, sea butterflies will change.
Despite their name, sea butterflies don’t fly at all: They’re silvery, swimming snails that flap wide, wing-like extensions of their feet to keep from sinking.
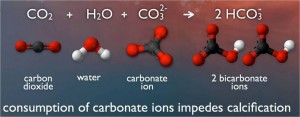
These snails build their protective shells from a mineral called calcium carbonate. Pieces of calcium carbonate tend to float freely in the sea, as charged molecules called carbonate ions. But when carbon dioxide dissolves into the sea, it starts a chemical reaction. The carbon dioxide and water molecules combine with calcium carbonate molecules. This produces molecules called bicarbonates.
Even though bicarbonate sounds like carbonate, it’s useless to animals like sea butterflies. They can’t build shells from bicarbonates. And the chemical reaction takes apart the calcium carbonate molecules the animals might have used. At the same time, greater amounts of carbon dioxide cause reactions that turn more and more carbonates into bicarbonates.
Like a rude party guest who devours your favorite snacks, carbon dioxide comes into the sea and takes away the carbonates.
When sea butterflies and other animals take calcium carbonate from the water and turn it into material for their shells, the process is called calcification. The shells that you can find on the beach were built by animals that used to live in them. And most of these shells are made of calcium carbonate.
Without carbonates, snails can’t build their shells. Without shells, bye bye snails. Carbon dioxide not only makes the oceans more acidic, it also reduces the supply of carbonates.
“Organisms that calcify will have more and more trouble calcifying,” says Jorge Sarmiento, who studies ocean changes at Princeton University. “That’s going to change things in the ocean.”
In a recent experiment, a team of scientists from France and Monaco compared the shells of two groups of sea butterflies. One group lived in normal ocean water. The other group lived in ocean water with extra carbon dioxide. After five days, sea butterflies living in normal water were better at building their shells than the sea butterflies living in the acidified water. Scientists predict that in 100 years, the oceans will have about the same amount of carbon dioxide as the acidified water in the experiment did. That means sea butterflies could have problems in the next century.
The carbonate problem may extend far beyond the cold seas: Tiny shelled creatures such as sea butterflies are at the bottom of the food chain, which means other animals eat these creatures to live. Those animals, in turn, provide food for other animals, which means the sea butterfly’s carbonate problems could potentially affect the lives of much larger animals.
Scientists are still trying to understand how carbonate changes will affect different species. Not all species will react the same: In 2008, studies showed that ocean acidification may even boost calcification rates in some animals.
The big question for organisms like sea butterflies, says Sarmiento, is, “What happens as you go to lower and lower carbonate ion concentrations? Do they die out or do they change?”
Double dose of trouble for coral
That’s a question also asked about coral, which usually forms reefs in warm, shallow waters near the equator. About one percent of the ocean floor is covered by coral reefs.
Imagine the ocean as a giant chessboard. All the coral reefs together wouldn’t occupy one square. That may not seem like much until you know that 25 percent of all life in the ocean depends on coral reefs. Corals grow and grow and grow: Coral reefs can be hundreds of years old, or older. Colorful fish, spiny anemones and shy octopi live on coral reefs — as do millions of other species.
Coral reefs sprawl across the ocean floor like multicolored forests, most with skeletons made of calcium carbonate — similar to the shells of the sea butterflies. And just as for sea butterflies, the carbonate shortage that comes with ocean acidification means trouble for coral reefs.
Despite their beauty and their skeletons, coral reefs are not invincible. In 2007, researchers grew two types of coral in acidified water and saw, firsthand, what could happen to coral in the future. After 12 months, the coral skeletons had completely dissolved in the modified water.
Not all was lost: The coral had left behind the tiny buds that are at the base of the skeleton. When the scientists returned the damaged coral to normal seawater, the skeleton grew back. This experiment shows that acidic water hurts coral reefs, but that coral might survive and regrow if conditions improve.

Climate change also boosts the average temperature of the oceans, posing another problem for coral. In warmer water, coral are more likely to develop infectious diseases. In the last 20 or 30 years, as the oceans have been warming, corals have been getting sicker, and more often.
For coral, more carbon dioxide delivers a double whammy: Warmer temperatures cause disease, and ocean acidification hurts coral skeletons. In addition, more carbon dioxide means fewer carbonates ― which means coral will have trouble building skeletons.
When a coral reef dies, its inhabitants must find new places to eat and live. The problem extends beyond the underwater world. Millions of people depend on coral reefs for their livelihoods — for food, work or tourism, for example. The disappearance of coral reefs wouldn’t just lead to the disappearance of many fish. It would change the way of life for people around the world.
Time for a U-turn
Experiments on coral reefs and sea butterflies hint at what the future may hold if more carbon dioxide ends up in the oceans. Other experiments suggest other species may also suffer: In more acidic water, sea urchins have trouble reproducing and are more likely to develop illnesses; squids and crabs have trouble breathing.
William Cheung, who works at the University of East Anglia in England, says we might expect to see fish get smaller, too. He and his colleagues built a computer model to predict how 5,000 fish species might change as the world gets warmer and the oceans get more acidic. The model predicted that more than 3,800 of those species will not grow as large in warmer, more acidic oceans. This change may happen because the fish have to use energy to get rid of acidic water, rather than to grow.
The problems could run all the way up the food chain: Reduce the menu for one animal, and you’ve reduced the menu for other animals, as well (fewer sea butterflies means hard times for the animals that eat sea butterflies). Neill points out, however, that ocean acidification can be reversed. The reactions that connect carbon dioxide to a reduction in carbonates can be stopped. If less carbon dioxide makes it into the water, the ocean will stop becoming more acidic, and calcium carbonate will be left available to the organisms that use it to build shells.
If people reduce the amount of carbon dioxide they put in the air, then less will end up in the ocean. The IPCC says that to avoid major destruction to the world’s oceans, by 2050 people must cut carbon dioxide emissions in half. (The IPCC is the International Panel on Climate Change. It was established by the United Nations and pulls together the latest science to try and determine how climate change may affect us).
Of course, cutting emissions by half is no easy task. Neill says he thinks it’s possible to avoid catastrophe in the ocean, but only if people start thinking about themselves as part of a larger system that includes the ocean. “If one of us [reduces our carbon-dioxide emissions], it doesn’t make much difference. If 1,000 of us do it, that’s still not much difference,” he says. “But if a million of us do it, if we are emulating the natural way, that’s the way the systems are designed to work as living organisms. That’s what community is.”







